Watch the video lecture of this topic for a better understanding
Carbon dioxide (CO2) has the potential to be sustainably converted back into useful fuels. Researchers at Lawrence Berkeley National Laboratory (Berkeley Lab) have improved the process’s selectivity by developing a new approach to modify the surface of the copper catalysts used to assist the reaction. They hope to improve the process to select for, or to yield, a higher amount of desirable carbon-rich products.
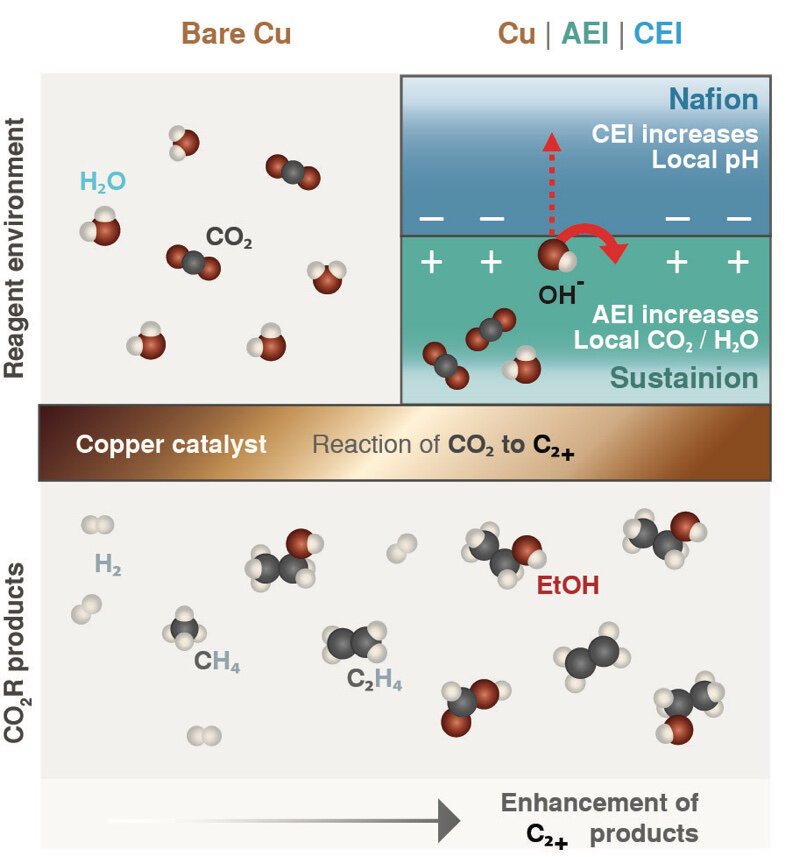
Berkeley Lab researchers have found that copper is the best catalyst for this reaction. But it doesn’t give high selectivity to the desired products, they say. “Our group has found that you can do various tricks with the local environment of the catalyst to provide that selectivity,” the scientist says.
The researchers had established the precise conditions that gave the best electrical and chemical environment for creating commercially interesting carbon-rich products. Those conditions are contrary to those that naturally occur in a typical fuel cell, which uses a water-based conductive material.
Bell and his team sought a design that could be used in the aqueous environment of fuel cells. They used thin layers of ionomers, polymers that allow certain charged molecules (ions) to pass through while excluding others.
The team hypothesized that the environment should modify the pH and amounts of water and CO2 in the catalyst’s immediate vicinity in a way that would steer the reaction to generate carbon-rich products that can be readily converted to useful chemicals and liquid fuels, Bell says.
Researchers applied a thin layer of each ionomer, as well as a bilayer of both ionomers, to copper films supported by a polymer material. They found that carbon-rich products accounted for 80% of the energy consumed by the reaction.
The researchers inserted a hand-sized electrochemical cell near one end of the cell to measure the total current flowing through the cell. They measured the gasses and liquids collected in adjoining reservoirs during the reaction to CO2 in a two-layer case.
The researchers used a pulsed voltage with the bilayer ionomer coating to increase CO2 and pH. They achieved a 250% increase in carbon-rich products compared to uncoated copper and a static voltage. The researchers concluded that the improved reaction was a consequence of the high CO2 concentration that built up in the coating layer immediately on top of the copper. That combination countered the concentration trade-off that tends to occur in the absence of the ionomer films, the researchers say.
Further information
More information: Chanyeon Kim et al, Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings, Nature Energy (2021). DOI: 10.1038/s41560-021-00920-8
Also read