Written by Adeel Abbas
Introduction to organic chemistry
Table of Contents
Knowing about fundamental principles of organic chemistry is much worthy. Because it is better to know about the priniciples of organic chemistry before diving in organic chemistry books.
Historical approach towards organic chemistry
In early days of chemistry the scientists were not able to distinguish between organic and inorganic compounds. In these days the comparison of organic and inorganic compound has become clearer.
Organic compound and vital force theory
It was thought by early chemist that organic compounds could be formed mainly in living organisms. Plants and animals are living organisms. Vital force is present in plants and animals. This vital force is necessary to synthesise these compounds in them.
The organic compound being obtained from the living organisms were called organic in nature. It was thought in possible to prepare any animal or Vegetable product in the laboratory.
Wohler’s approach towards organic chemistry
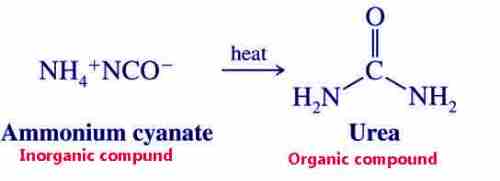
In 1828 Wohler prepared urea by heating ammonium cyanate in the laboratory. Urea is an organic compound found in animal urine.
Hence the definition of chemistry changed. When urea was synthesized in the laboratory there happened revolution in the minds of the chemists. In the following, the syntheses of many organic compounds were achieved. By 1850 the vital force theory was finally disapproved.
Modern definition of organic chemistry
The study of compounds containing carbon is called organic chemistry. It became evident to the scientist that most of the compounds formed by the living self-contained carbon for the definition were shifted from origin to composition.
Important elements present in organic compound
Carbon is not the only element present in the organic compound. But carbon is an essential element present in organic compound most of these organic compound also contains hydrogen. The following is the list of other compounds that may be present in the organic compounds.
- Oxygen
- Sulfur
- Nitrogen
- Phosphorus
- Halogens
- Metals
So we can define organic chemistry in another way that the branch of science that deals with the study of compounds containing carbon and hydrogen ( O, S, N, P, Halogens, Metals) is called organic chemistry.
Modern definition of Organic chemistry
Compounds of carbon which are not organic in nature
There are certain compounds that contain carbon but they are not organic in nature. Following is the list of important compounds which have carbon in them but they are not called organic compounds because they do not have functional groups in them.
- Carbon monoxide
- Carbon dioxide
- CS2
- Carbonates of metals
- Hydrogen cyanide
- Bicarbonates of metals
- Methyl cyanide
- Metal carbides
The chemical properties of the above-mentioned come carbon compounds are quite different from the properties of the organic compounds.
Some properties of organic compounds
The organic compound over the fundamental laws of chemistry as inorganic compound do. Compounds are studied as a separate branch of chemistry because of the following reason.
1 Peculiar nature of Carbon (catenation)
Carbon being a 6th element of the periodic table has a property to link with other carbon atoms this property is called catenation carbon can form long chains and rings. It can produce single and multiple born with carbon-nitrogen Sulphur and oxygen.
Due to this behavior carbon can form numerous compounds of various sizes shapes and structures. There are about 5 million organic compounds present now. If we combine all the compounds of elements present in the periodic table, only carbon has a greater number of compounds than all of these. Therefore catenation is a very important property of carbon.
2 Non- ionic character of organic compounds
As we know that electronegativity of carbon is 2.5. It is neither very high Nor very low. Therefore it gives covalent compounds and not the ionic compound. The reactions given in organic compounds are different from those of ionic compounds.
3 similarity in behavior
Organic compounds are divided into various categories it depends upon the the type of functional group present in them. So there are homeo logos series according to the families of organic compound.
Every member of the homologous series has similar behaviour. For example, in the case of alkane with to carbon will behave the same as in the case of carbon-containing three are four carbon atoms.
It means their chemical properties will remain the same to some extent moving forward in a homologous series from compounds containing one carbon atom to more carbon atoms.
4 Complexity of organic compound
Organic compounds are much larger in size. Their structures are complex. For example, the formula of starch is (C6 H10 O5 )n. It means that thousands of C6 H10 O5 units are linked with each other to form one molecule of starch. Similarly, proteins are also very complex molecules. Their molecular masses range from a few thousand to a million.
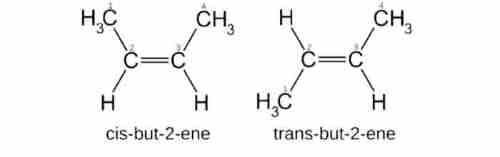
5 isomerism in organic compounds
The compounds with the same chemical formula and different structural formulas are called isomers. And this process is called isomerisam.
It is probably the most important property of organic compounds that give rise to such a healing number of organic compounds. Isomerism an organic compound. This property of organic compound increases the number and variety of organic compounds.
6 Rate of organic reactions
Reactions related to organic compounds are slow. The reason behind this is that bonds which are being broken and which are being formed are covalent in nature.
7 solubility of organic compound
Most of the organic compounds are not water salute. They are soluble in organic solvents. For example sum of important organic solvents are listed below.
- Ether
- Benzene
- Acetone
- Methanol
- Carbon tetrachloride
- Petroleum Ether
- Ethanol
- Chloroform
- Ethyl acetate
Importance of organic chemistry e in daily life activity
Organic chemistry is closely related to the activities of our daily life. Let us take up the necessities of human beings in our life. We observe that these necessities are related to organic chemistry in a way a larger extent.
Following points indicates the importance of Chemistry in daily life activities
- The food we eat is organic in nature
- The food goes to our bodies and the organic chemical reaction takes place in our body
- Metabolism growth and maintenance of our body functions involve organic chemistry
- A similar type of changes of metabolism growth and maintenance is present in plants and
- All of our wearing including the cotton cloth synthetic fibers and shoes are organic substances
- The material which is used for cleanliness like soap and detergents are also an organic compound
- The structural material used in our houses for decoration like Paints and varnishes are all organic in nature
- The thing used in our vehicles like fuels and lubricants are organic substances
- All type of medicine is used in allopathy involve the organic compounds
- Insecticides light DDT which are being widely used are organic substances
- Hormones and steroids are complex organic compounds present in our body to maintain the different functions of our body
- Paper and things are the sources of Civilization and organic material
- Perfume and flavors and all cosmetics are also organic in nature
- Propellants explosives and refrigerants are well known organic materials
From all the above-mentioned points, we can understand how important organic chemistry is in our daily life activities.
Share this with others