In this article, the author has explained about…
- Analysis of compound
- Molecular and Empirical Formula
- Combustion analysis
- Percentage of elements in a compound
Analysis of a compound
Table of Contents
To find the molecular formula of a compound following steps are considered.
- All the elements present in the compound are identified. This is called qualitative analysis.
- The mass of each element is determined in the compound. This is called quantitative analysis.
- The mass of each element is used to calculate the percentage by mass of each element.
- The percentage is used to determine the empirical formula of the compound
- Finally, the molecular formula is obtained from empirical formula and molecular mass.
Percentage of an element in a compound
It is the number of grams of an element present in 100 g of the compound.
The percentage from the given amount: The percentage of each element in a compound can be determined by the following formula.
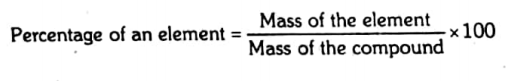
The percentage from formula mass: The percentage of each element in a compound can be determined theoretically from the formula mass of a compound.

Empirical formula
The formula which shows the simplest whole-number ratio between atoms of a compound is called the empirical formula.
Definition of Empirical formula
For example, the empirical formula of Hydrogen peroxide is HO. It shows that the simplest whole-number ratio between H and O is 1:1.
Steps involved while determining the empirical formula
- First of all, determine the percentage composition of each element in a substance.
- Then divide the percentage of each element by its atomic mass to get a number of gram atoms or moles.
- After this divide the moles of each element by the smallest number of moles to get atomic ratios.
- If the atomic ratio is not in a simple whole number then multiply with a small suitable number to get the whole-number ratio.
- Thus empirical formula is obtained.
Also read: Experimental techniques in chemistry
Molecular formula
The formula which shows the exact number of atoms of each element present in one molecule of a compound is called the molecular formula.
Definition of Molecular formula
It is based on an actual molecule.
For example in the case of Molecular formulas of benzene is C6H6 and Glucose C6H12O6.
The link between Empirical and Molecular formula
The molecular formula may be the multiple of the empirical formula. For many compounds empirical and molecular formulas are different.
For example, the empirical formula of benzene and glucose are CH and CH2O respectively. However, their molecular formulas are C6H6 and C6H12O6 respectively.
This relationship can be expressed as
Molecular formula = n (empirical formula)
Where in the above equation n is an integer and its value is 1, 2, 3…
The n shows the ratio of molecular mass and empirical formula mass.
n = Molecular mass / Empirical formula mass
The compounds may have the same empirical and molecular formulas because for such compounds the value of n is unity.
For example NH3, H2O, CO2, C12H22O11, etc.
Comparison between Empirical and Molecular formula
| Empirical Formula | Molecular Formula |
| It shows the simplest whole-number ratio between atoms of a compound | It shows the exact number of atoms of each element present in one molecule of a compound |
| The empirical formula is shown by both ionic and covalent compounds | Molecular compounds do not show molecular formula |
| It is based on a formula unit that may or may not exist independently | It is based on an actual molecule that exists independently |
| It is obtained from the percentage composition of elements i.e. chemical analysis | It is obtained by multiplying n with the empirical formula |
| Example: NaCl, CH2O, CH are empirical formulae of sodium chloride, glucose, and benzene respectively. | Example: C6H12O6 and C6H6 are molecular formulae of glucose and benzene respectively. |
Empirical formula from combustion analysis
Combustion analysis
It is an experimental technique by which amounts of various elements present in the given amount of a compound are determined by burning.
Definition of combustion analysis
Organic compounds containing only Carbon, hydrogen, and oxygen are analyzed by combustion analysis.
The procedure of Combustion analysis
- First of all, a weighed quantity of the compound is burned in a combustion tube that is fitted in a furnace.
- To start burning of compound oxygen is supplied.
- Hydrogen and carbon present in the compound are converted into water and carbon dioxide.
C & H in sample + O2 ——————-> CO2 + H2O
Schematic diagram of combustion analysis
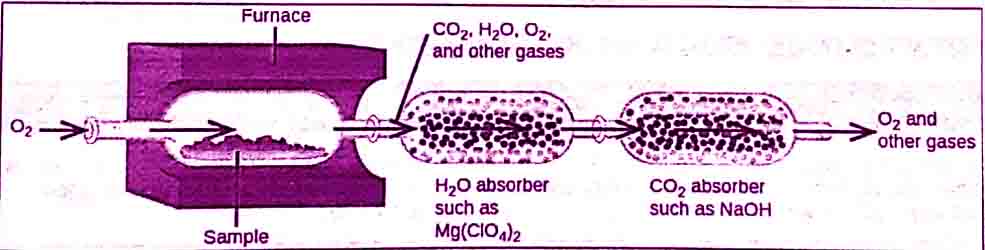
In combustion analysis, the water vapors formed are absorbed in magnesium perchlorate [Mg(ClO4)2].
The CO2 produced is absorbed in a 50% KOH solution.
The increase in masses of these absorbers gives the masses of H2O and CO2 produced.
Other absorbers can be used for other products of combustion.
The amount of oxygen is determined by the method of difference.
Formulas for calculation of CH and O in a given compound
Solved examples
Example No:1
8.657 g of a compound were decomposed into its elements and gave 5.217 g of carbon, 0.962 g of hydrogen, 2.478 g of oxygen. Calculate the percentage composition of the compound under study.
Solution:
Mass of compound= 8.657 g
Mass of carbon= 5.217 g
Mass of hydrogen= 0.962 g
% age composition=?
Let us apply the formula
Percentage of C= Mass of carbon/Mass of compound x 100
Putting the values % age of C= 5.217/8.567 x 100 = 60.26
Now let us apply the same formula for Hydrogen
% age of H= 0.962/8.657 x 100 = 11.11
Apply the same formula for Oxygen
% age of O= 2.478/8.657 x 100 = 28.62
This result tells us that in one hundred grams of the given compound there are 60.26 g of carbon, 11.11 g of hydrogen, and 28.62 g of oxygen. The sum of these percentages is 100.
Example No:2
Ascorbic acid which is known as vitamin C contains 40.92 % carbon, 4.58 % hydrogen, and 54.5 % of oxygen by mass. What is the empirical formula of ascorbic acid?
Solution:
Mass of Carbon= 40.92 %
Mass of hydrogen= 4.58 %
Mass of Oxygen= 54.5 %
Empirical formula=?
There are four steps to calculate the empirical formula.
Let us discuss it one by one.
Step 1: BY dividing the % ages by atomic masses of the elements, to get moles of each element.
Moles of carbon= 40.92/12.01 = 3.407
Moles of Hydrogen= 4.58 g/1.00 g = 4.54
Moles of oxygen= 54.5 g / 16 gmol-1 = 3.406
Step 2: To get simple whole number ratio of atoms, divide above number of moles by the least number such as 3.406:
Therefore,
C= 3.407 / 3.406 = 1
H= 4.54 / 3.406 = 1.33
O= 3.406 / 3.406 = 1
Step 3: Convert these values into the whole numbers by multiplying with 3.
C:H:O = 1:1.3:3:1
C:H:O = 3(1:1,33:1) = 3:4:3
Step 4: Atomic ratios of elements give the empirical formula for ascorbic acid such as C3H4O3.
Let others know about this