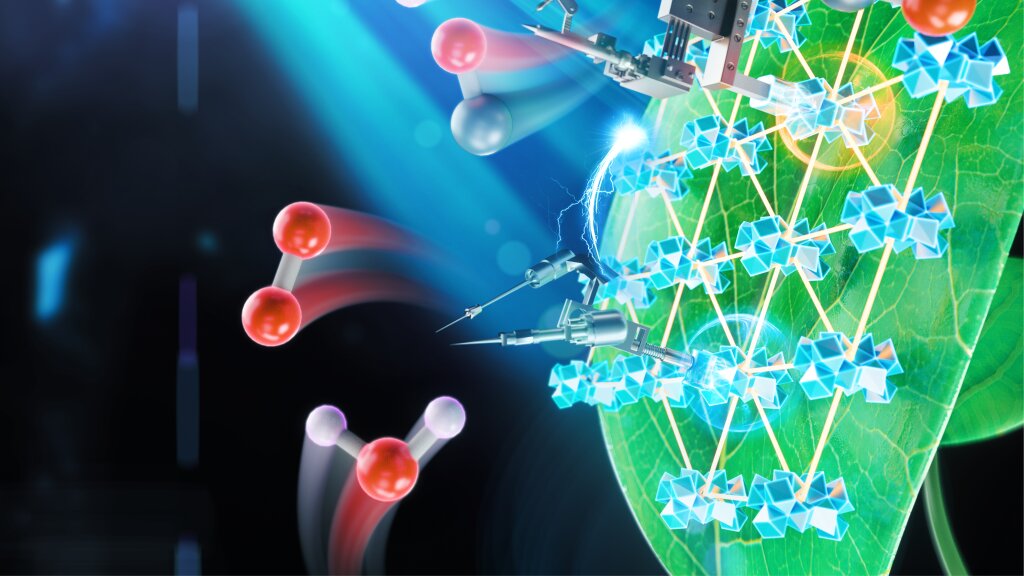
A Nature Catalysis study conducted at the University of Chicago showed an innovative new system for artificial photosynthesis that is more productive than previous artificial systems by an order of magnitude.
Artificial Photosynthesis would be useful for producing methane for use as a fuel and for generating electricity. Artificial photosynthesis would be useful for producing ethanol and other fuels.
For the past two centuries, we have depended on fossil fuels for concentrated energy; hundreds of millions of years of photosynthesis have been compressed into a convenient, energy-dense substance.
We have limited access to fossil fuels, and fossil fuel use is a key contributor to global warming and ongoing climate change.
“The biggest challenge most people don’t realize is that even nature has no solution for the amount of energy we use.
Even photosynthesis isn’t quite as good as we have become accustomed to over the past few decades.
This book discusses a variety of options for getting power from the sun. One of those options involves “artificial photosynthesis,” which engineers are developing right now.
The chemical equipment in a single leaf of the plant is extremely complex, and it’s not easy to use it for our own purposes.
This research looks promising, but there is still a lot to learn before this becomes a reliable alternative fuel.
“This is a huge improvement on existing systems, but just as importantly, we were able to lay out a very clear understanding of how this artificial system works at the molecular level, which has not been accomplished before.
The many different types of proteins and pigments that make up these assemblies allow plants to perform a wide range of functions such as photosynthesis, growth, and reproduction.
Carbon is the main building block of all life forms on Earth. Life is composed of billions of molecules called proteins, and proteins are made up of hundreds of millions of carbon atoms strung together.
Scientists have been trying to find the “holy grail” of energy production—a sustainable source that doesn’t involve burning fossil fuels—but it’s hard to come up with a
The team started with a type of material called a metal-organic framework or MOF, a class of compounds made up of metal ions held together by an organic linking molecule.
That’s why they designed the MOFs as a single layer, to give it the maximum surface area for chemical reactions. They put everything in a solution that included a cobalt compound to carry electrons around.
Finally, they found amino acids that work well in moisturizers. And they discovered what combinations work best.
They were able to make improvements to both halves of the reaction: the process that breaks apart water and the one that adds electrons and protons to carbon dioxide.
Both of these two sentences can be made into passive sentences, by adding the word “help” before the word “reaction.
The breakthrough may also be applied to other chemical reactions, including those in pharmaceuticals, nylon and other products. You need to make a lot of material, but a very small quantity of some molecules, such as the starting materials to make pharmaceutical drugs and nylons, among others, could be very useful.
More information
Guangxu Lan et al, Biomimetic active sites on monolayered metal–organic frameworks for artificial photosynthesis, Nature Catalysis (2022). DOI: 10.1038/s41929-022-00865-5
More from Latest chemistry news
Latest Chemistry Research News
- Beer hops compounds could help protect against Alzheimer’s disease
- Scientists found method for extending shelf life of fresh pasta by 30 days
- Discovery of biodegradable commercial paper for sensing food temperature and freshness
- Stars have much more time to form planets-Recent research claims
- New study found USA facing severe Heat waves in rivers