Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means that isotopes have the same chemical properties, but they may have different physical properties, such as mass and radioactivity.
Hydrogen, carbon, oxygen, nitrogen, chlorine, iron, uranium, potassium, iodine, and lead all have isotopes.
Examples of Isotopes
Table of Contents
Here are a few Examples of Isotopes:
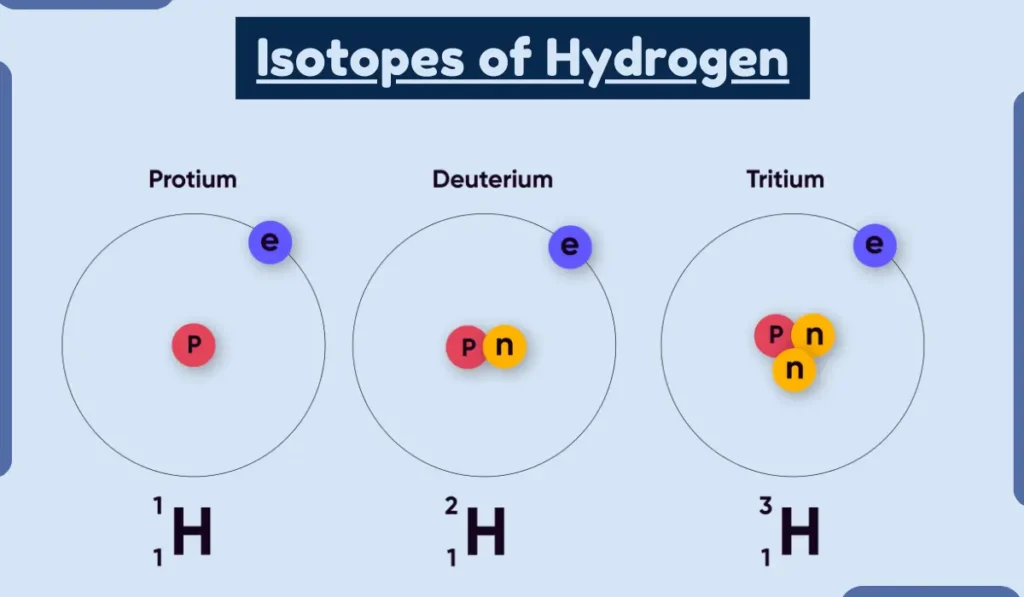
1. Hydrogen
Hydrogen has three isotopes: protium, deuterium, and tritium. Protium is the most common isotope of hydrogen, and it has no neutrons. Deuterium has one neutron, and tritium has two neutrons.
2. Carbon
Carbon has three isotopes: carbon-12, carbon-13, and carbon-14. Carbon-12 is the most common isotope of carbon, and it has six neutrons. Carbon-13 has seven neutrons, and carbon-14 has eight neutrons.
3. Oxygen
Oxygen has three isotopes: oxygen-16, oxygen-17, and oxygen-18. Oxygen-16 is the most common isotope of oxygen, and it has eight neutrons. Oxygen-17 has nine neutrons, and oxygen-18 has ten neutrons.
4. Nitrogen
Nitrogen has two isotopes: nitrogen-14 and nitrogen-15. Nitrogen-14 is the most common isotope of nitrogen, and it has seven neutrons. Nitrogen-15 has eight neutrons.
5. Chlorine
Chlorine has two isotopes: chlorine-35 and chlorine-37. Chlorine-35 is the most common isotope of chlorine, and it has 18 neutrons. Chlorine-37 has 20 neutrons.
6. Iron
Iron has four isotopes: iron-54, iron-56, iron-57, and iron-58. Iron-56 is the most common isotope of iron, and it has 30 neutrons. Iron-54 has 28 neutrons, iron-57 has 31 neutrons, and iron-58 has 32 neutrons.
7. Uranium
Uranium has three isotopes: uranium-234, uranium-235, and uranium-238. Uranium-238 is the most common isotope of uranium, and it has 146 neutrons. Uranium-235 has 143 neutrons, and uranium-234 has 142 neutrons.
8. Potassium
Potassium has three isotopes: potassium-39, potassium-40, and potassium-41. Potassium-39 is the most common isotope of potassium, and it has 20 neutrons. Potassium-40 has 21 neutrons, and potassium-41 has 22 neutrons.
9. Iodine
Iodine has 37 isotopes, but only one stable isotope, iodine-127. The other 36 isotopes are radioactive.
10. Lead
Lead has four stable isotopes: lead-204, lead-206, lead-207, and lead-208. Lead-208 is the most common isotope of lead, and it has 126 neutrons. Lead-204 has 122 neutrons, lead-206 has 124 neutrons, and lead-207 has 125 neutrons.
Also Read:
| Examples of Sublimation | Examples of Lipids | Examples of Mixtures that can separated through separatory funnel |
| Examples of Acids | Examples of Chemical Change | Examples of Homogenoues Mixtures |
Good