Written by Adeel Abbas
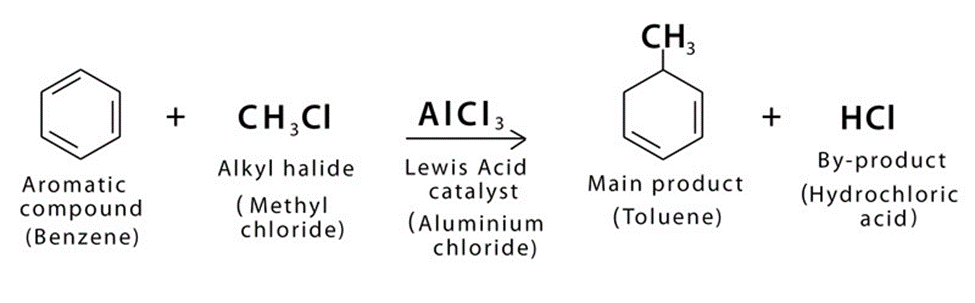
The introduction of alkyl group (R) into benzene is called Friedel-Craft Alkylation.
Friedel crafts alkylation is important reaction of benzene.
Overall Reaction For Friedel Crafts Alkylation
Table of Contents
Explanation
The term Friedel-Craft alkylation describes the substitution of an alkyl group for an aromatic proton.
This is accomplished by using a carbocation to conduct an electrophilic assault on the aromatic ring. Alkyl halides are used as reactants in the Friedel-Craft Alkylation process, which produces alkyl benzene.
Catalyst
Friedel-Craft Alkylation takes place in the presence of aluminum chloride (AlCl3) or ferric chloride (FeCl3). Aluminum chloride or ferric chloride acts as a catalyst in this reaction.
Mechanism
Three steps make up the Friedel-Craft Alkylation reaction’s mechanism.
Step 1: Formation of carbocation
The reaction between the alkyl halide and the Lewis acid catalyst (AlCl3) produces an electrophilic carbocation.
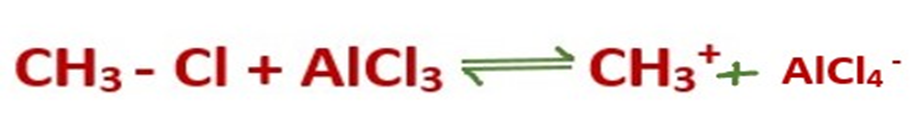
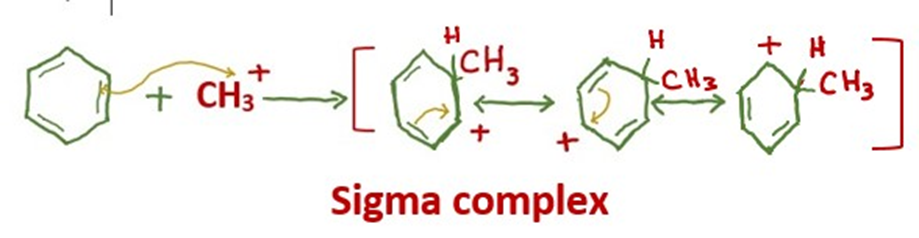
Step 2: Electrophilic attack forms a sigma complex
The carbocation proceeds to attack the aromatic ring, forming a cyclohexadienyl cation as an intermediate. The aromaticity of the benzene is temporarily lost due to the breakage of the carbon-carbon double bond.
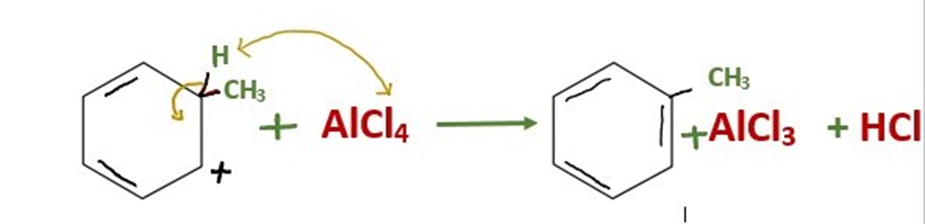
Step 3: Loss of proton regenerates the aromatic ring and gives the alkylated product
The intermediate is deprotonated, which causes the carbon-carbon double bond to regenerate and give the chemical its aromaticity again. The hydrochloric acid that is created by this proton goes on to regenerate the AlCl3 catalyst.
Addition of ethyl group to prepare Ethyl Benzene
Addition of ethyl group into benzene ring is Friedel Crafts alkylation reaction. As a result of this reaction ethyl benzene is prepared.
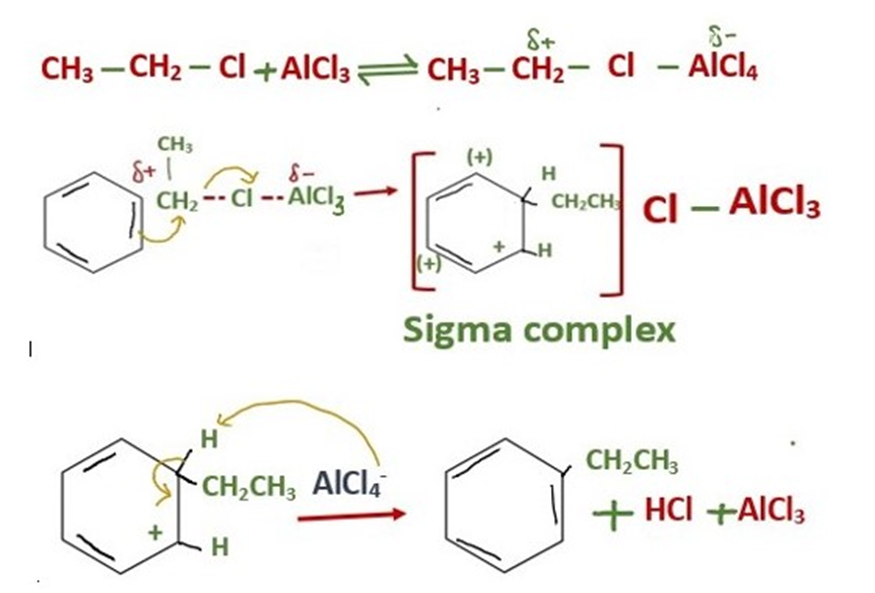
The mechanism of addition of ethyl group into benzene is same as we discuss above.
With primary alkyl halides, the free primary carbocation is too unstable. The actual electrophile is a complex of aluminum chloride with the alkyl halide.
In this complex, the carbon-halogen bond is weakened and there is considerable positive charge on the carbon atom. The mechanism for the aluminum chloride-catalyzed reaction of ethyl chloride with benzene is as follows.
Limitations of the Friedel-Crafts Alkylation Reaction
Some important limitations of Friedel-Crafts alkylation are listed below.
- Aryl and vinyl halides cannot be employed in this process due to the exceedingly unstable carbonations they produce.
- The catalyst may become inactive as a result of complex formation if the aromatic ring contains a deactivating group (such as an NH2 group).
- In order to prevent polyalkylation in these reactions, an excess of the aromatic component must be utilized (addition of more than one alkyl group to the aromatic compound).
- Aromatic compounds that are not involved in the Friedel-Crafts alkylation reaction are less reactive than mono-halo benzenes.
It is significant to note that, as with any reaction involving carbonations, this reaction is susceptible to carbocation rearrangements.
FAQs
What is the purpose of Friedel-Crafts alkylation lab?
What Purposes Does Friedel-Crafts Alkylation Serve? The formation of C-C bonds and the activation of C-H are two of organic chemistry’s most crucial applications of Friedel-Crafts reactions. Friedel-Crafts style alkylation’s serve as the building blocks for the creation of a wide range of industrial goods by attaching an alkyl group to an arene molecule.
Why is it called alkylation?
Transferring an alkyl group from one molecule to another is known as alkylation. An alkyl carbocation, a free radical, a carbanion, or a carbine is possible transitions for the alkyl group (or their equivalents).