LEARNING OBJECTIVES
In this article, author has explained what are monosaccharides, structural properties of monosaccharides, stereochemistry of monosaccharides and chemical reactions of monosaccharides.
Monosaccharides are poly-hydroxy-aldehydes or ketones. Monosaccharides are the smallest unit of carbohydrates. When monosaccharides are linked through a glycosidic bond, oligosaccharides or polysaccharides are formed.
Glucose and fructose are common monosaccharides.
Monosaccharides are simple carbohydrates present in food which include glucose, fructose, galactose, mannose, and ribose. They are classified into two categories – dietary and non-dietary monosaccharides. Dietary monosaccharides include glucose, fructose, and galactose. Non-dietary monosaccharides include mannose, ribose, and xylose.
They are important for human health as they are needed for energy production in the body. They are also used as energy substrates to produce glycogen, fats, and nucleic acids. The main function of monosaccharides is the storage of carbohydrates in the liver.
Non-dietary monosaccharides are also found in some drugs such as Lidocaine and Dantrolene.
Dietary monosaccharides are used as food components and are also essential for the synthesis of glycogen, lipids, and proteins. Glucose and fructose are the primary forms of dietary monosaccharides. Glucose is the primary source of energy in the human body.
Glucose is metabolized in the liver to glycogen which is a major form of stored carbohydrate in the body. Glycogen is the source of energy in the muscles. Fructose is an additional sugar present in fruits and vegetables.
Structural features of monosaccharides
Table of Contents
Stereoisomerism
Carbon which is attached to four different groups is called “asymmetric carbon”. Asymmetric carbon is the basis of stereoisomerism. The monosaccharides which possess “asymmetric carbon” show stereoisomerism. The number of isomers can be calculated by the simple formula 2n where n refers to the number of asymmetric carbon.
Let’s take an example of monosaccharide triose, glyceraldehyde.
Glyceraldehyde has one asymmetric carbon.
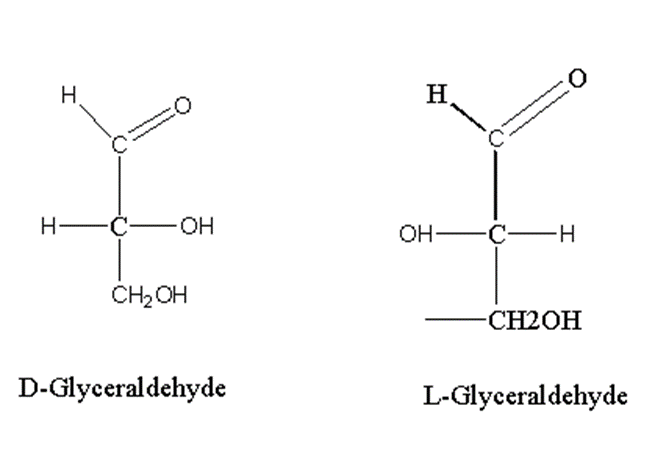
If the OH group adjacent to alcohol is present on the right side, it is D-isomer while L-isomer has an OH group on the left side. Glyceraldehyde has one asymmetric carbon so the number of isomers is 2.
All monosaccharides show stereoisomerism except dihydroxyacetone.
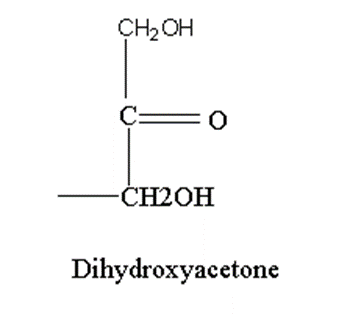
Dihydroxyacetone does not have asymmetric carbon therefore, it does not possess stereoisomerism.
Enantiomers
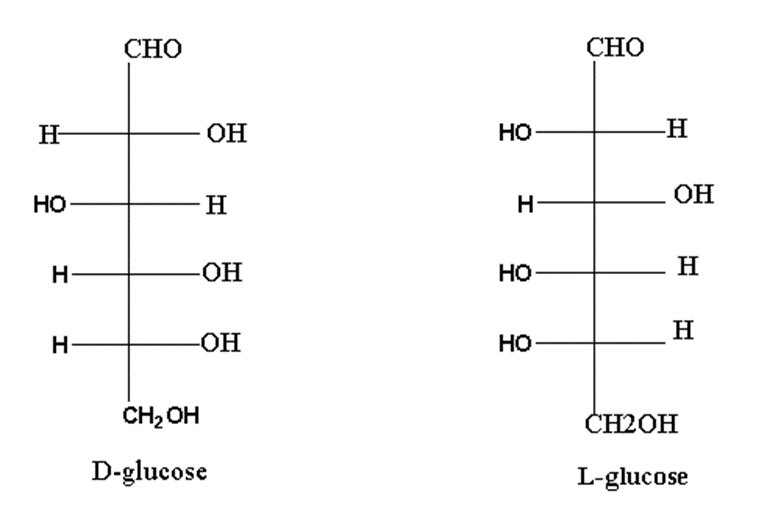
Mirror images of monosaccharides are called enantiomers.
For example, D and L glucose are enantiomers of each other.
Optical isomerism in monosaccharides
When asymmetric carbon is present in a compound, it can show optical activity. When light passes through the solution of the optical isomer, it can rotate light either in the right or left direction.
When light is rotated in the right direction, it is called dextrorotatory (+). When light is rotated in the left direction, it is called levorotatory (-). So, stereoisomers of monosaccharides can be D (+), D (-), L (+), and L (-).
For example, glucose is dextrotatory in solution.
Epimers of monosaccharides
Two compounds are called epimers of each other when they possess the same molecular formula but different spatial configurations of attached groups around a single carbon atom.
For exanple, D-glucose and D-mannose are epimers of each other.
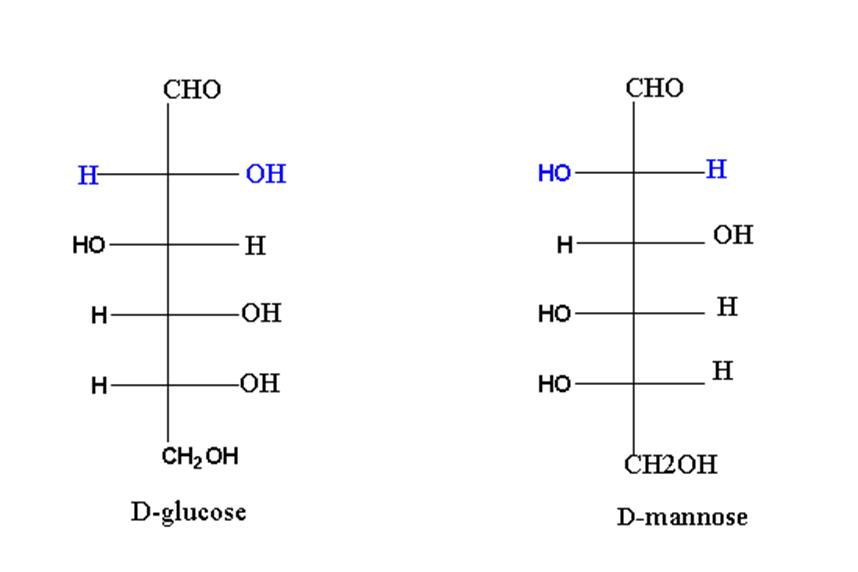
As clear from the structure, there is a different arrangement of groups at carbon 2. So, glucose and mannose are called epimers of each other.
Similarly, glucose and galactose are C4 epimers.
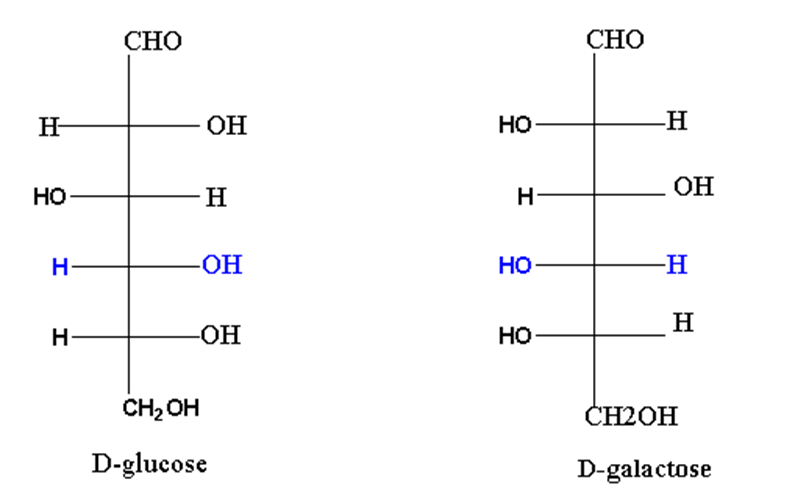
Aldose and ketose isomerism
Same molecular formula but have different functional groups (Aldo or keto group) are called aldose ketose isomers of each other.
For example, glucose and fructose. Glucose is aldohexose while fructose is ketohexose.
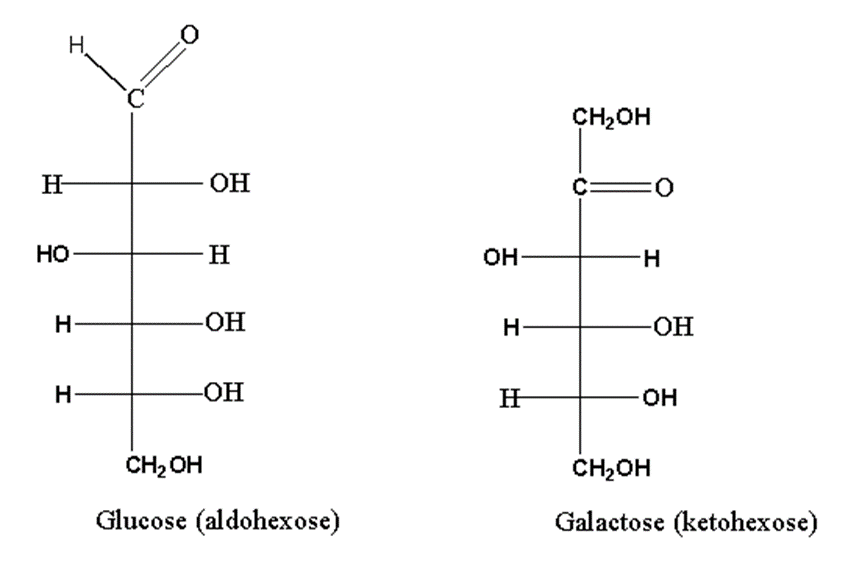
As you can see, both monosaccharides differ concerning functional groups. Glucose has an Aldo group while fructose has a keto group.
Anomers of monosaccharides
In solution, monosaccharides are present in a ring structure. When the spatial configuration of monosaccharides is different at one carbon in the ring structure, they are called anomers of each other.
For example, α-D-glucose and β-D-glucose.
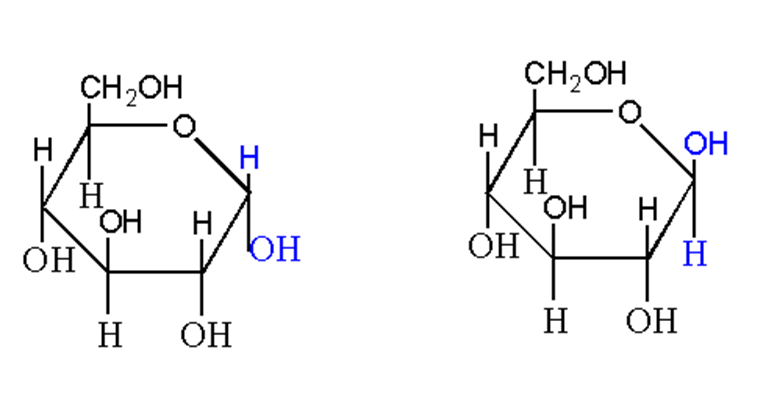
There is a change in configuration at carbon 1 known as anomeric carbon. So, they are called alpha and beta anomers of glucose.
Reactions of monosaccharides
1. Tautomerization/enolization
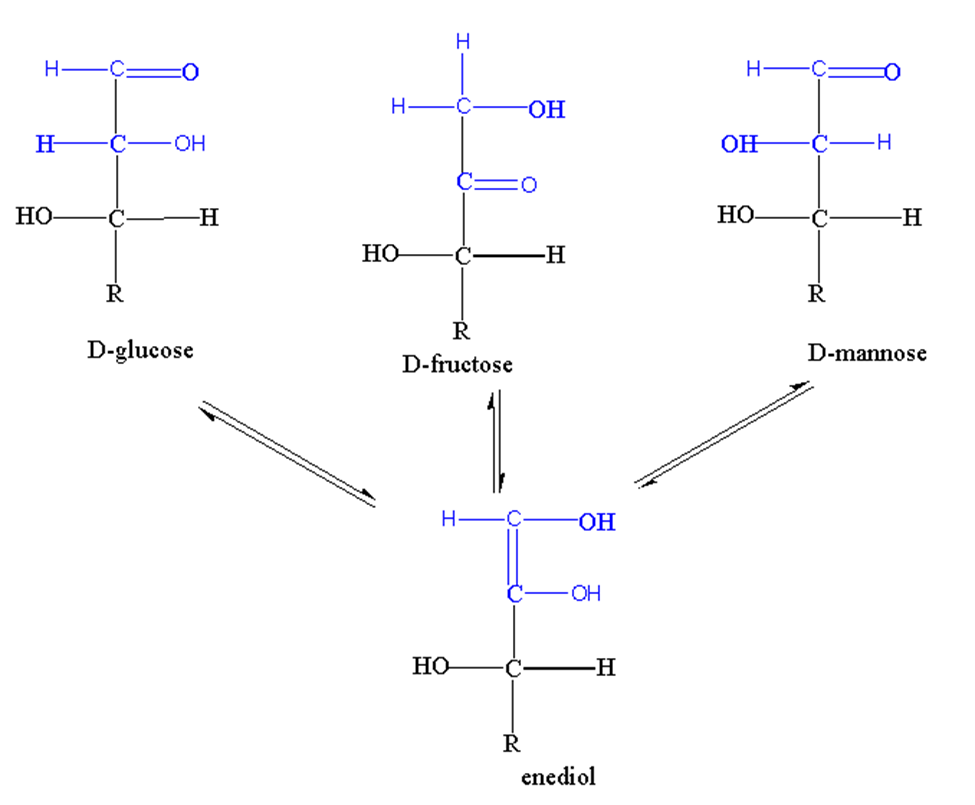
When hydrogen transfers from one carbon atom to another, it is called Tautomerization.
The process of Tautomerization produces enediols. The monosaccharides which possess anomeric carbon undergo the process of Tautomerization when present in alkaline solutions.
Let’s take the example of glucose. When glucose is present in an alkaline solution, isomers are formed like D-fructose, D-glucose, and D-mannose. These isomers have one thing in common which is ennediols. Ennediol is the intermediate of isomerization of these three monosaccharides.
2. Oxidation of monosaccharides
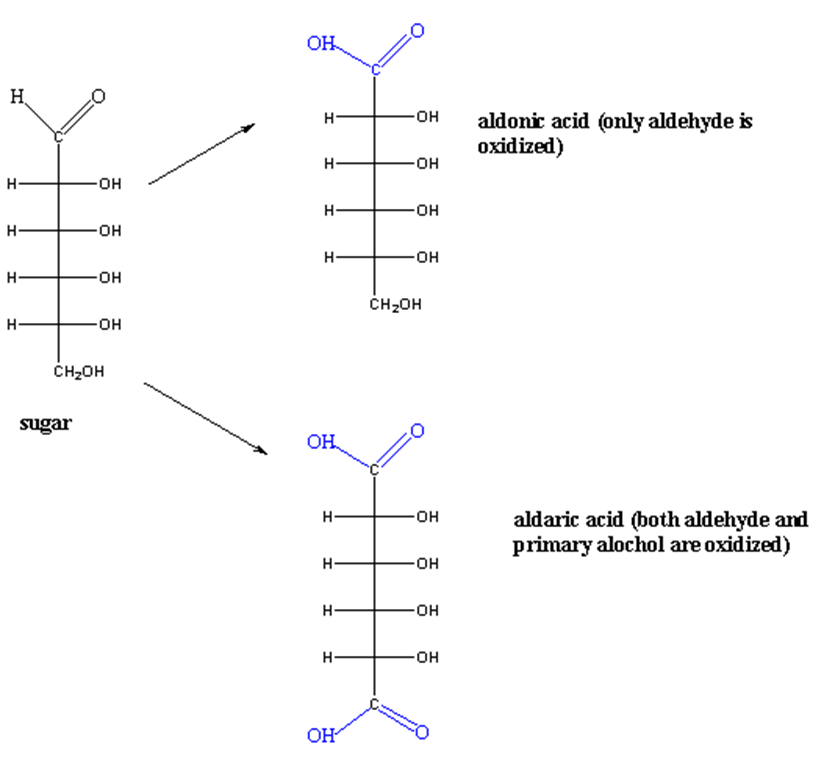
Aldoses have two functional groups; aldehyde and alcohol. Both can undergo oxidation. The oxidation of monosaccharides produces carboxylic acid.
If only the aldehyde group is oxidized, gluconic acid is formed.
CHO —> COOH
If the alcohol group is oxidized, glucuronic acid is formed.
CH2OH —> COOH
If the oxidizing agent is strong, both groups (aldehyde and alcohol) are oxidized to aldonic and aldaric acid respectively.
Reduction in monosaccharides
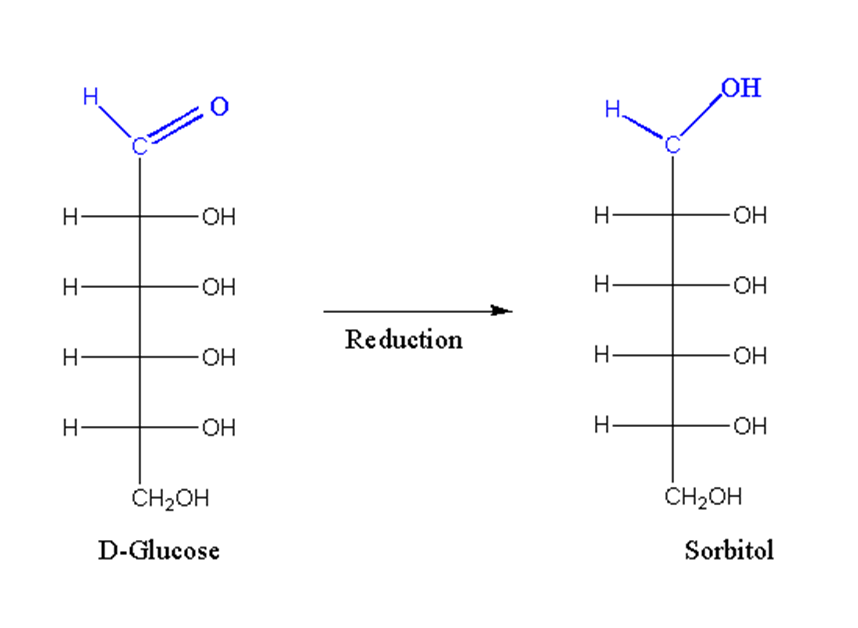
When treated with a strong reducing agent, the aldehyde or keto group is reduced to an alcohol.
For example, D-glucose is reduced by D-sorbitol.
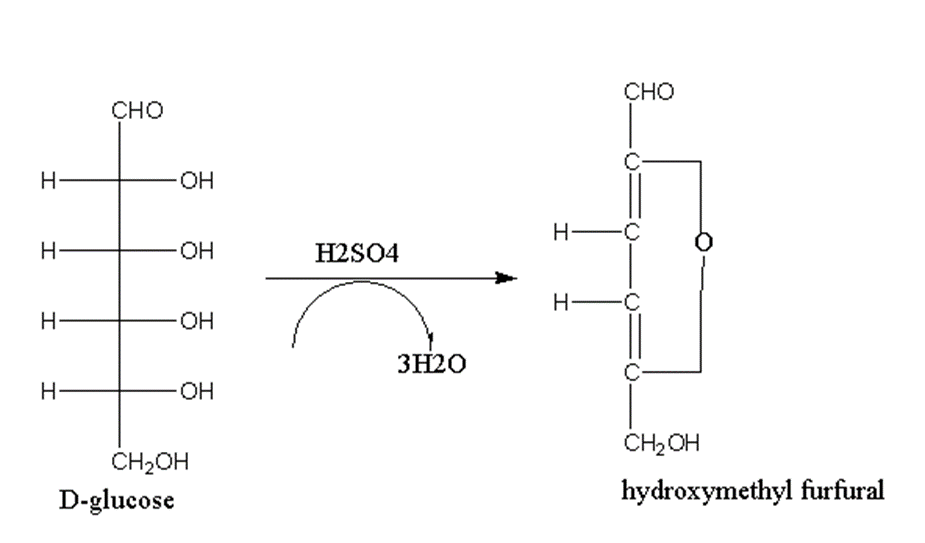
Dehydration of monosaccharides
When monosaccharides are treated with concentrated sulfuric acid, they undergo dehydration with the removal of three water molecules.
For example, when D-glucose is treated with sulfuric acid it gives hydroxymethyl furfural.