Written By Adeel Abbas
Definition
Table of Contents
Those groups that direct incoming groups to meta position are called meta-directing groups.
We can also define it as “Electron withdrawing groups in the aromatic ring are called meta-directing groups.”
For better understanding also read my article on ortho-para directing groups.
Examples of Meta directing groups
Some of the important meta directing groups are –CN, NO2 -COOH, -CHO, -COR etc.
Because these are electrons withdrawing groups they attract the electrons to a benzene ring. Benzene becomes electron deficient at the ortho and para positions. Therefore incoming electrophile attaches at the meta position.
By drawing the benzene ring’s electrons away toward themselves, these groups reduce the electrons’ availability. The chemical reactivity of benzene is thereby reduced.
Due to the electron-withdrawing effect of such substituents, the ortho and para positions have become more electron deficient than the meta position.
Thus the incoming electrophile will prefer to attack the meta position rather than the ortho and para position.
How electron withdrawing groups are meta directing
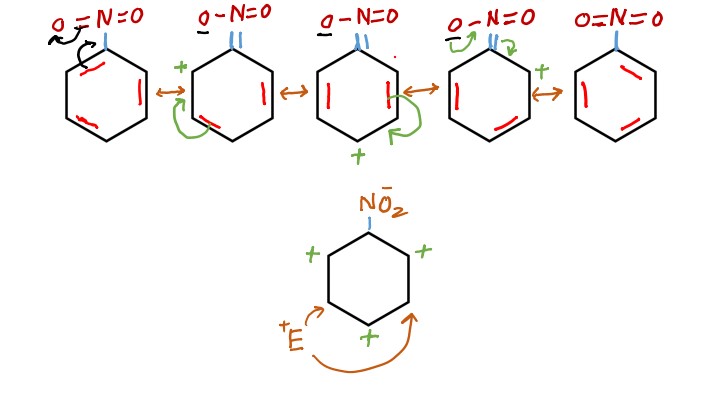
It is cleared from above mechanism how meta directing groups directs the incoming electrophile at meta position.
Examples of Meta directing groups
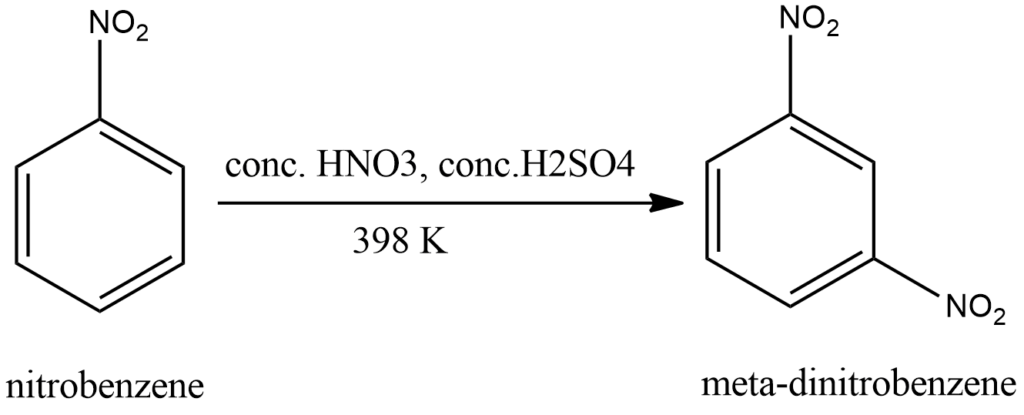
Nitration of nitrobenzene
As you can see in below example of nitration of nitrobenzene that nitro being an electron withdrawing group directs another nitro group on meta position.
Nitration of cyanobenzene
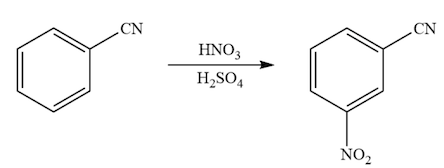
Cyano group is also an electron withdrawing group and directs the nitro group at meta position.