Learning objectives
In this article, the author has explained what are oxides, the types and classification of oxides, and variation in the periodic table.
Oxides
Table of Contents
Binary compounds of oxygen with elements of the periodic table are called oxides.
Definition of oxides
Examples of oxides
Na2O, CaO, Al2O3, CO2, NO2, Cl2O3, OF2 etc are all oxides.
Types of oxides
There are three types of oxides on the basis of acidic properties.
- Basic oxides
- Acidic oxides
- Amphoteric oxides
Basic oxides
Those oxides which yield bases in water and are mostly made up of metal oxides are known as basic oxides.
Definition of basic oxides
The elements of group I-A and II-A groups except Be and the elements of the II-A group except Zn from basic oxides.
Whenever they are dissolved in water, these give bases. With the increases in metallic character of the elements down the group the oxides of the elements of the same group become more and more basic. CO2 and SiO2 are acidic oxides. GeO2 and SnO2 are amphoteric oxides while PbO2 is basic oxides.
Acidic oxides
Those oxides which when dissolved in water give the acidic solution are known as acidic oxides.
Definition of acidic oxides
Oxides of carbon, nitrogen and sulphur are acidic in nature. CO2, NO2, SO2 are acidic oxides.
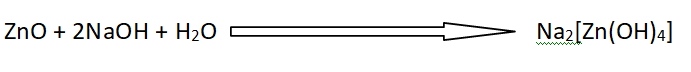
Amphoteric oxides
Those oxides which have acidic and basic character both are called amphoteric oxides.
Definition of amphoteric oxides
Amohoteric oxides behave as acids towards a strong base. They act bases towards a strong acid.
For example:
ZnO reacts with H+ ions to give Zn+2 ion and behaves as a basic oxide.

ZnO also reacts with OH– ions
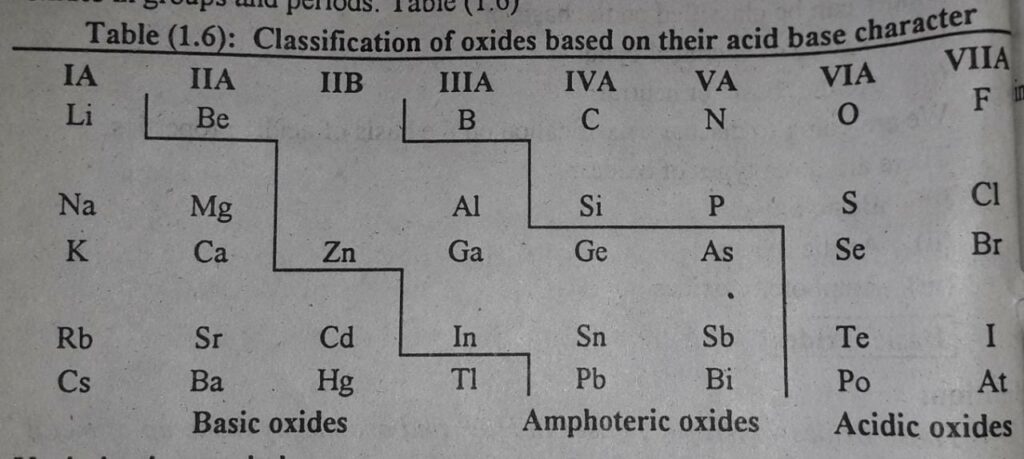
Variation of the nature of oxides in the periodic table
There is an almost systematic variation of the acidic and basic character of the oxides in groups and periods in the periodic table.
Following table gives the idea about classification of the oxides on the basis of acid base character.
Variation of nature of oxides in a period
When we move from left to right in a period, the oxides become more and mmore acidic in nature. Look at the gradual increase of change in the nature of the oxides of third period.
| Group | I-A | II-A | III-A | IV-A | V-A | VI-A | VII-A |
| Oxides | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl2O7 |
| Nature | Strongly basic | Basic | Amphoteric | Acidic | More acidic | Even more acidic | Strongly acidic |
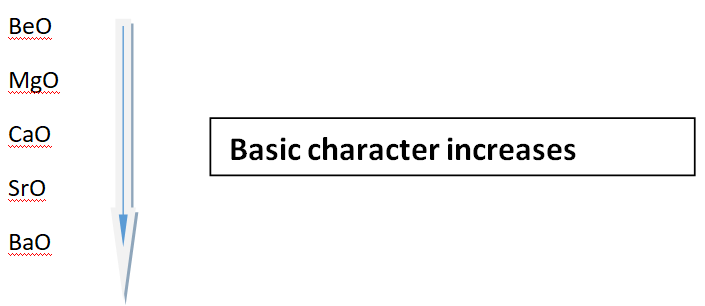
Variation of nature of oxides in a group
When we move from top to downward direction in a group; an increase in basic character oxides is observed. Look at the oxides of II-A group elements. BeO is least basic, while BaO has maximum basic character.
Group II-A
Oxidation state of the metal and its effect on the nature of oxides
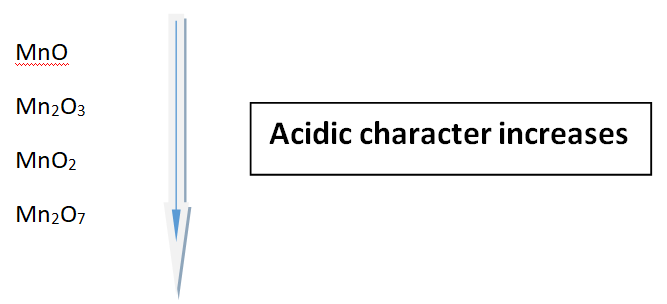
There are certain metals that have variable oxidation states. Therefore, these metal gives various oxides. This can be explained by saying; greater the oxidation number of the metal, the greater the acidic character of the oxides.
Let us look at the oxides of Manganese (Mn)
Oxides of Manganese (Mn)
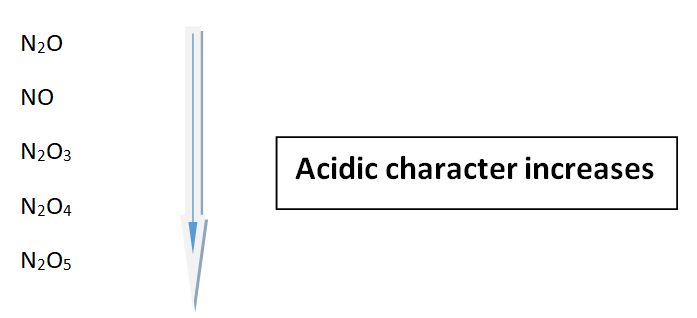
Oxides of nitrogen
These are five oxides of nitrogen and their acidic strengths increase with the increasing oxidation state of nitrogen.
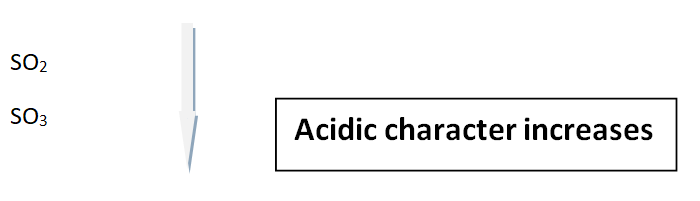
Oxides of Sulphur
Similarly, the oxides of Sulphur have the same trends as above.