Introduction
Table of Contents
pH meters are indispensable tools in the world of science and industry, allowing us to measure the acidity or alkalinity of substances with precision. In this article, we will explore the working principle, components, calibration, and applications of pH meters.

I. Understanding pH
pH is a measure of the acidity or alkalinity of a substance. It is based on the concentration of hydrogen ions (H+) present in the solution. The pH scale ranges from 0 to 14, where 0 indicates strong acidity, 7 represents neutrality, and 14 signifies strong alkalinity.
II. Working Principle of pH Meters
pH meters operate on the principle of potentiometry, where they measure the voltage difference between a reference electrode and a glass electrode. The process involves the following steps:
1. Electrodes
pH meters consist of two main electrodes:
a. Glass Electrode
The glass electrode is the heart of a pH meter. It consists of a thin glass membrane that is sensitive to changes in hydrogen ion concentration. When immersed in a solution, the glass membrane interacts with H+ ions, generating a potential difference.
b. Reference Electrode
The reference electrode provides a stable reference voltage against which the glass electrode potential is measured. It is typically a silver-silver chloride electrode immersed in a potassium chloride solution.
2. Calibration
Before use, pH meters must be calibrated using buffer solutions of known pH values. This calibration ensures accurate and reliable measurements. Buffer solutions with pH values corresponding to the range of interest are used to adjust the meter’s readings.
3. Measurement
Once calibrated, the glass electrode is immersed in the sample solution. The potential difference between the glass and reference electrodes is measured and converted into a pH value using the instrument’s internal circuitry.
III. Components of a pH Meter
A pH meter typically consists of the following components:
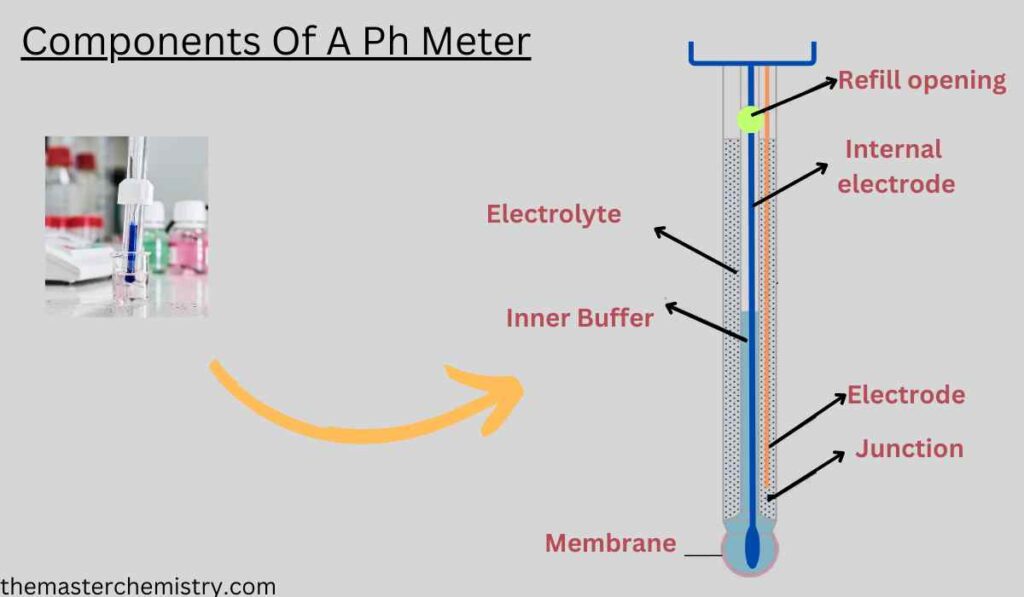
1. Main Unit
The main unit houses the circuitry, display, and controls of the pH meter. It allows users to set parameters, read measurements, and perform various operations.
2. Electrode Assembly
The electrode assembly comprises the glass electrode and the reference electrode. It is detachable for ease of maintenance and replacement.
3. Calibration Controls
pH meters are equipped with calibration controls that allow users to adjust the meter’s readings based on the buffer solutions used for calibration.
4. Temperature Compensation
To ensure accurate pH measurements, many pH meters have temperature compensation capabilities. Temperature sensors or probes are incorporated to compensate for the effect of temperature on pH values.
IV. Calibration and Maintenance
Regular calibration and proper maintenance are essential for accurate pH measurements. pH meters should be recalibrated periodically using fresh buffer solutions. The electrodes need proper cleaning and storage to prevent contamination and maintain their performance.
V. Applications of pH Meters
pH meters find applications in various fields:
1. Environmental Monitoring
pH measurements are crucial in environmental studies, particularly in water quality assessment, soil analysis, and monitoring of aquatic ecosystems.
2. Chemical Analysis
In laboratories, pH meters are used for chemical analysis, titrations, and quality control of products in industries such as food and beverage, pharmaceuticals, and cosmetics.
3. Agriculture and Hydroponics
pH meters play a vital role in agriculture and hydroponics, helping farmers and growers monitor and adjust the pH levels of soil and nutrient solutions for optimal plant growth.
4. Swimming Pools and Aquariums
Maintaining the proper pH balance is essential for the health and safety of swimming pools and aquariums. pH meters are used to monitor and regulate the pH levels in these settings.
pH meters are invaluable tools for measuring acidity and alkalinity accurately. By understanding their working principle, components, calibration procedures, and diverse applications, we can appreciate the significance of pH meters in various scientific, industrial, and environmental endeavors. Whether it’s ensuring water quality, optimizing chemical processes, or promoting healthy plant growth, pH meters are essential for maintaining balance and understanding the secrets of acidity and alkalinity.