A team of researchers affiliated with a number of institutions in China and one in the U.K. has used Raman spectroscopy to study water on Pd single-crystal surfaces. In their paper published in Nature, they describe how interfacial dynamics are related to interface structure as well as what was learned from studying it all so close.
Scientists have been working for years in the lab to find a way that would allow them access between hydrogen and oxygen. Now with modern inventions, they’ve finally found it! Matthias Waegele from Boston College recently published an article about this research.
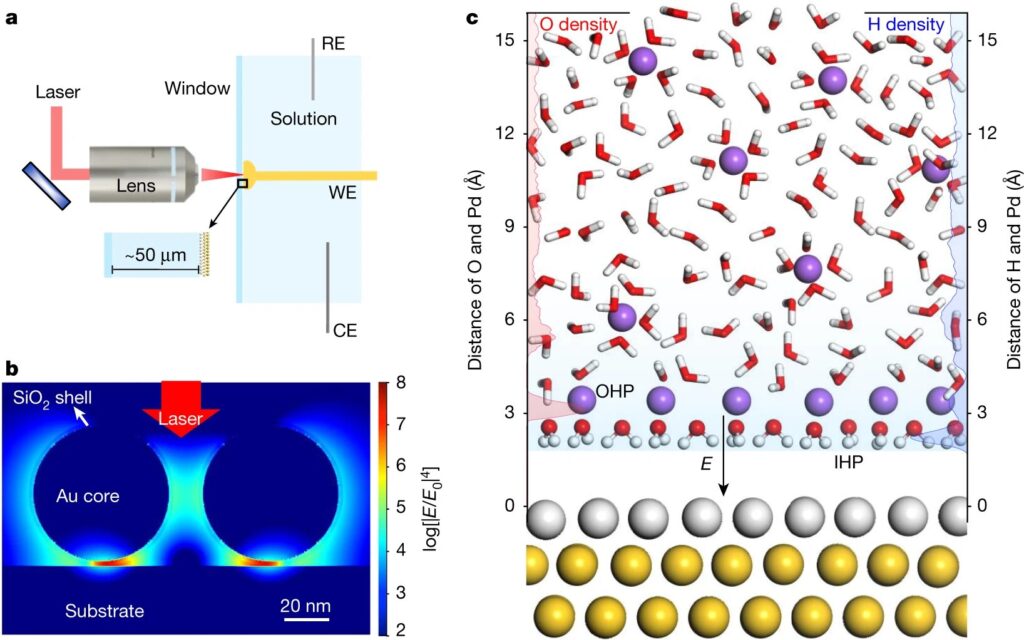
Scientists have discovered that water, when it forms a layer on top of another substance can be used as an excellent catalyst for certain transformations. This new research has found out how this happens at the solid-liquid interface and expanded on previous studies by using flat metal electrodes (palladium atoms sandwiching gold in between) which acted like “catalysts.”
Read More
Researchers discovered that they could use the technique in order to investigate more deeply into interfacial water samples. This is an amazing breakthrough.
Researchers have been able to use a network of water molecules, called hydration shells around an electrode at low sodium ion concentrations. They also found that when there are higher levels of this element present in the solution it becomes attracted and draws closer toward itself through hydrogen bonding which leads then on from being stuck together by palladium ions (which can be used for catalytic purposes).
The faster electron transfer rates between these two entities eventually result in splitting up both parts-hydroxide ions as well as H2O molecule themselves so they don’t get too close or create any issues with corrosion.
Further information
Yao-Hui Wang et al, In situ Raman spectroscopy, reveals the structure and dissociation of interfacial water, Nature (2021). DOI: 10.1038/s41586-021-04068-z