LEARNING OBJECTIVES
Table of Contents
In this article, the author has explained An in-depth guide on What is electrochemistry.
Electrochemistry is a type of chemistry that studies the reactions between electricity and chemicals.
It employs electrochemical cells to produce electric currents from chemical reactions. In this post, we will discuss..
- What is Electrochemistry
- Principle of electrochemistry
- Different Types of Electrochemical Cells
- Applications of Electrochemistry
- Historical Timeline for Electromechanical Devices
- Applications of electrochemistry in everyday life
- Safety considerations for working with electrolytes and electrodes
What is Electrochemistry?
Electrochemistry is the study of chemical reactions that produce electricity, or conversely those that take place because of electric current.
This branch of chemistry has led to some important discoveries about the nature and behavior of atoms and molecules during these changes in charge. It can be helpful for understanding how batteries work but also helps us understand corrosion, corrosion, and other chemical processes.
Principle of electrochemistry
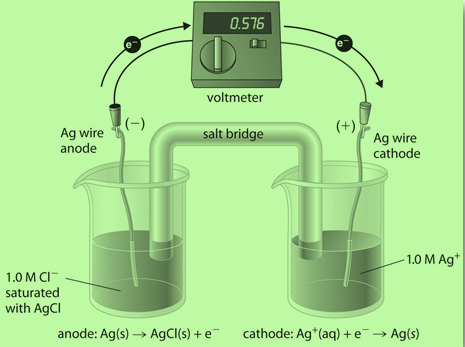
The principle of electrochemistry deals with how changes in voltage will affect the chemical reactions that happen at an electrode. This is one of the most important concepts to understand when studying chemistry and provides a basis for many different types of experiments.
For example, if you were to use this principle to create an experiment where your goal was to find out what happens when you increase voltages on electrodes, then you would choose two electrodes made from different materials (metal vs metal or metal vs liquid).
You would then apply a specified voltage between each pair and watch what happened over time as they reacted with each other. These experiments are usually done in order to study corrosion or oxidation rates at different currents or voltages.
History of electrochemistry
An important part of electrochemistry is its history. Even before the discovery and naming of metals such as gold, silver, copper; there was a great understanding that certain materials were capable of producing electricity when in contact with one another. As early as 600 BC it became known that rubbing fur on amber would cause this effect to occur (this is why the electron in Greek means amber).
This was also around the time that Thales of Miletus conducted studies on what materials were capable of producing electricity. He discovered through his experiments, that if he rubbed a piece of silk against amber that it produced static sparks. The Greeks named this phenomena ‘electron’ which means amber.
This was a very important discovery within the world of electrochemistry and its history because it opened up an understanding that, through the process of rubbing two materials together, electricity could be produced in this manner. This allowed for future discoveries to be made on how these processes worked. In 1660 Otto von Guericke created what is known as a ‘Magdeburg Hemispheres’.
This device caused controversy within the scientific community by showing that, when separated from one another with air in between them, they were capable of producing electricity and it wouldn’t be until 1729 before definitive proof was given through the experiments conducted by Stephen Gray on this phenomena.
The modern work in electrochemistry can be traced back to the 19th century when it was discovered that there were two different types of electricity. This discovery allowed for a greater understanding of electrochemistry and its history as scientists sought out ways in which they could create either type of electricity from one another through chemical means.
Different Types of Electrochemical Cells
An electrochemical cell is a device that converts chemical energy into electrical energy. Some examples of cells are the zinc-carbon battery, lead accumulator battery and alkaline battery.
An electrolytic cell uses spontaneous redox reactions to create flow of current through an external circuit, while in a galvanic or voltaic cell, a voltage is applied across the electrodes of the cell to drive an electric current through an external circuit.
There are many different types of electrochemical cells that use various combinations and concentrations of chemicals in order to create electricity for energy storage.
Galvanic or Voltaic Cells
Anode Reaction (oxidation) and Cathode Reaction (reduction) for Electrolytic Cells.
Zinc Carbon Battery, Lead Accumulator Battery, Alkaline Battery are all examples of electrochemical cells.
Applications of electrochemistry in everyday life
Electrochemistry is the study of chemical changes that happen in a solution when electrical energy passes through it. Electrochemical reactions can be used to find out what substances are present in an unknown sample, or they may be used to change one substance into another. For example, electroplating involves coating metal objects with thin layers of silver or gold.
Uses of electrochemistry in batteries
Batteries are an everyday example of how electrochemistry can be used to change one substance into another. Batteries use chemical reactions between substances like zinc and copper, or lead and acid, to produce electricity.
One type of battery is the alkaline battery which contains a mixture of manganese dioxide (MnO) and zinc powder in a steel can. When the battery is put into use, one half of the manganese dioxide (MnO) chemical dissolves to form Mn ions while each atom loses an electron. The resulting electrons travel through the circuit to produce electricity for our devices.
Uses of electrochemistry in electroplating
Electroplating is a process that uses an electric current to reduce dissolved metal cations so that they form a thin coherent metal coating on an electrode. The term is also used for electrical oxidation of anions onto a solid substrate, as in the formation of silver chloride electrodes from silver nitrate solution using potassium hexacyanoferrate(III) as the anode.
Uses of electrochemistry in electroforming
Electroforms can be used for artistic purposes, such as those by Andy Goldsworthy and jewelers like Samuel Kirkland Lothrop. An example of a commercially important electroform is silverware that has been plated with nickel or rhodium.
Uses of electrochemistry in corrosion protection
Electrochemistry is used in corrosion protection as well, such as:
Metal coating on the hull of ships to protect against salt water and other corrosive substances
Chemical process where electrodes are dipped into a solution containing metal ions, using electricity to deposit or remove metal from an electrode surface. This is most commonly applied with chromium plating.
Electrochemical process in which metal ions entering an electrolyte is reduced to the metallic state at one electrode (the cathode) and oxidized to ionic form at another electrode (anode).
Uses of electrochemistry in medicine
There are many applications of electrochemistry in medicine. Electrochemical sensors are used to monitor the hydrogen ions concentration inside cells, pH levels for certain diseases, or electrolyte balance in blood and urine samples. This is very useful because they help diagnose human disease conditions such as diabetes mellitus, kidney failure, etc. Another example would be cardiac pacemakers which are implanted to regulate the heartbeat.
Safety considerations for working on electrochemistry
Safety considerations for working on electrochemistry are very important. This is especially true if you have a large cell or are using high voltages in your lab. Here I will outline the main safety points to consider when doing experiments with ionic liquids, batteries, and fuel cells.
The most important care that should be taken while dealing with electrochemical cells is to avoid any direct electrical connection with the working electrode. This is especially important in systems like lithium-ion batteries and supercapacitors that can deliver high currents (hundreds of amps or even >1000A).
In larger cells, such as those used for electrolysis experiments, it is wise to check whether they are able to withstand accidental short-circuits.
In addition to the electrical hazards, any chemical reaction that might release toxic fumes (for example SOFCs) or flammable solvents should be avoided and appropriate ventilation provided. Safety glasses and gloves must also be worn at all times during lab work.
Finally, do not forget about the proper disposal of used chemicals – lithium-ion batteries contain potentially dangerous chemicals and need to be recycled.
Conclusion
Electrochemistry is an important branch of science that has wide-ranging applications in everyday life. The next time you need to charge your cell phone or get rid of rust on a metal surface, remember the basics of electrochemical reactions!
Do you have any questions about this post? Leave me a comment below and I’ll do my best to answer them for you.