Nomenclature is the systematic process of naming chemical compounds in a way that accurately and precisely describes their composition, structure, and properties. It is an essential aspect of chemistry that allows scientists, researchers, educators, and other professionals to communicate effectively and unambiguously about various substances. Nomenclature provides a common language for discussing and categorizing a wide range of chemical compounds, enabling clear communication and understanding within the field of chemistry.
Importance of Nomenclature
Table of Contents
The diversity of aliphatic hydrocarbons necessitates a standardized naming system that can convey important structural information about the compounds. A systematic nomenclature ensures that chemists worldwide can understand a compound’s structure and properties just by looking at its name.
Common or Trivial Names
Common names are often simple and easy to remember, which makes them convenient for casual conversation, non-scientific contexts, and situations where precise chemical information is not crucial. However, they can sometimes be ambiguous or misleading, especially when different compounds share the same name in various regions or languages. This lack of standardization and potential for confusion is why the IUPAC nomenclature system was developed to provide a systematic and unambiguous way of naming compounds.
In the early days, the compounds were named on the basis of
- Their history.
- The method of preparation.
- Name of the person working on it.
Examples
- The name Marsh Gas was given to methane because it was found in marshy places.
- Acetic acid derives its name vinegar (Latin, acetum means vinegar).
- Organic compounds, like barbituric acid after Barbara, were named after a person.
Such a system may have a certain charm but is never manageable.
Straight and branched chain Hydrocarbons
For alkanes with five or more carbon atoms, the root word is derived from the Greek or Latin numerals indicating the number of carbon atoms in a molecule, and the name is completed by adding ‘ane’ as a suffix, e.g. pentane (C5H12), hexane (C6H14), heptane (C7H16), etc.
The common or trivial names are applicable to all isomers of a given molecular formula. The prefixes n, iso, neo, are, however, to differentiate between isomers.
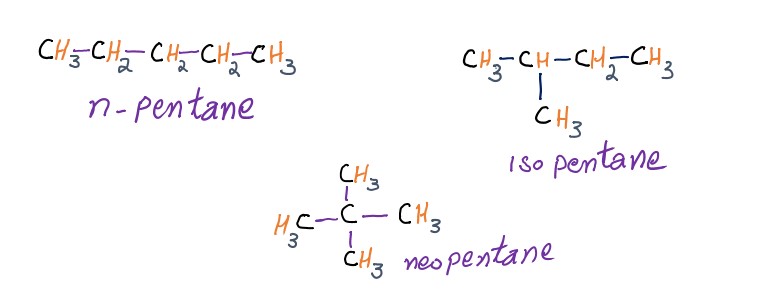
These prefixes have only limited use, as they are not workable with complex molecules. Moreover, common names give only a minimum of information about the structure of the compounds.
Examples
IUPAC Names
The IUPAC (International Union of Pure and Applied Chemistry) nomenclature system is a set of rules and guidelines used to systematically name chemical compounds, particularly organic and inorganic compounds, in a standardized and consistent manner. It was developed by the IUPAC to ensure that chemical compounds are named in a way that accurately reflects their composition, structure, and functional groups, regardless of language or geographical location. The IUPAC nomenclature system plays a critical role in promoting clear communication and understanding within the global scientific community.
- In 1889 the solution for naming the organic compounds systematically was sought by the International Chemical Congress.
- A report was accepted in 1892 in Geneva but it was found incomplete.
- In 1930 International Union of Chemistry (IUC) gave a modified report which is also referred to as Liege Rules. This report was further modified by the International Union of Pure and Applied Chemistry (IUPAC) in the year 1947. Since that date, the union has issued periodic reports on rules for the systematic nomenclature of organic compounds.
- The most recent report which was published in the year 1979.