LEARNING OBJECTIVES
In this article, the author has explained 7 Difference Between Vaporization And Evaporation.
When we talk about vaporization and evaporation, we are talking about the process of turning a liquid into a gas. Vaporization is the process of changing a liquid into its gaseous form by heating it. Evaporation is the process of changing water or other liquids to their vapor phase without any external heat source.
Watch the video lecture for better understanding of the topic
Vaporization and evaporation are two similar processes, but they have different properties that make them useful in different ways. The main difference between these two processes is that vaporizing requires an external energy source to provide enough heat for the change from liquid to gas, whereas evaporation does not need any outside input as long as there’s a temperature gradient between the system and its surroundings.
It can also be noted that while both methods convert one type of substance into another, only vaporization changes a liquid into a gas; evaporating simply changes a liquid into its vapor phase.
7 Differences between Vaporization and Evaporation
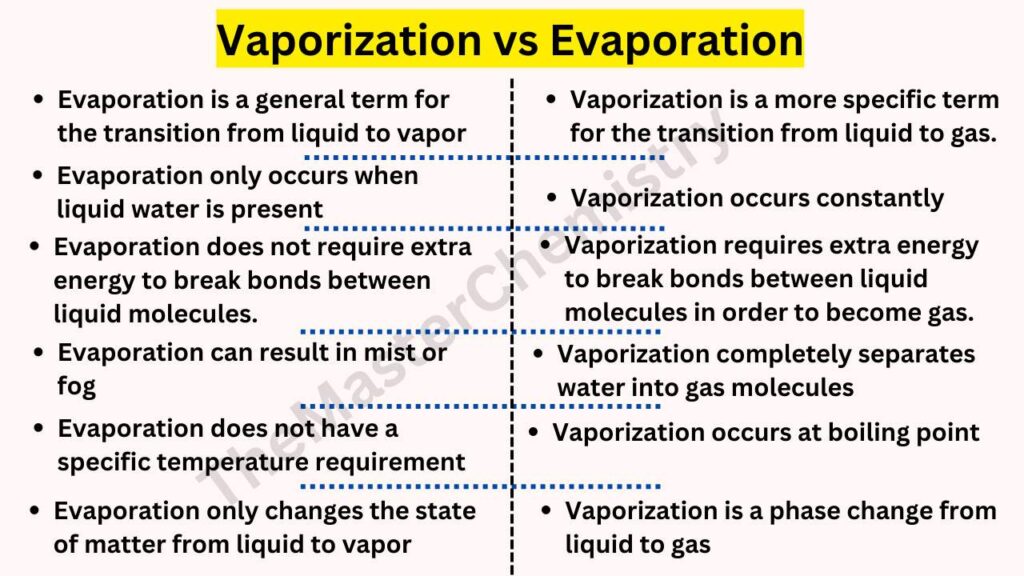
- The main difference between Vaporization and Evaporation is that Vaporization is a process that occurs at all times, but Evaporation only happens when there’s liquid water.
- The second difference between Vaporization and Evaporation is that with Vaporization it takes energy to break the bonds of liquid molecules in order for them to become vapor (gas), while Evaporation doesn’t take any extra energy.
- The third difference between Vaporization and Evaporation is that with Vaporization, the water molecules are completely separated into gas molecules, while with Evaporation some of the liquid vaporizes into a mist or fog.
- The fourth Difference between Vaporization and Evaporation is that vaporization happens when water is heated to its boiling point.
- The fifth difference between Vaporization and Evaporation is Vaporization occurs at a lower temperature than evaporation. The high heat used when evaporating water is not needed in vaporizing, making it more efficient and cost-effective.
- The sixth Difference between Vaporization and Evaporation is that vaporization is more specific than evaporation.
- The seventh Difference between Vaporization and Evaporation is that Vaporization is a phase change from liquid to gas whereas Evaporation only changes the state of matter from liquid to vapor.
Conclusion paragraph
Vaporization and evaporation are both processes that work to change the state of a liquid. They have different properties, though, which make them useful in different situations. If you’re looking for more information about vaporization or evaporation, contact us today! We can discuss how each process works and what they might be used for so you can get the most out of your time with us.