Written by Adeel Abbas
Solar-powered synthesis gas could recycle carbon dioxide into fuels and useful chemicals. The professor of electrical and computer engineering at the University of Michigan, who led the study, said it will reduce CO2 emissions.
syngas is usually derived from fossil fuels with the help of electricity and is composed of hydrogen and carbon monoxide. Toxic chemicals are often added to the process to make it more efficient.
“Our new process is actually pretty simple, but it’s exciting because it’s not toxic, it’s sustainable and it’s very cost-effective,” said the first author of the study, a Ph.D. student in electrical and computer engineering.
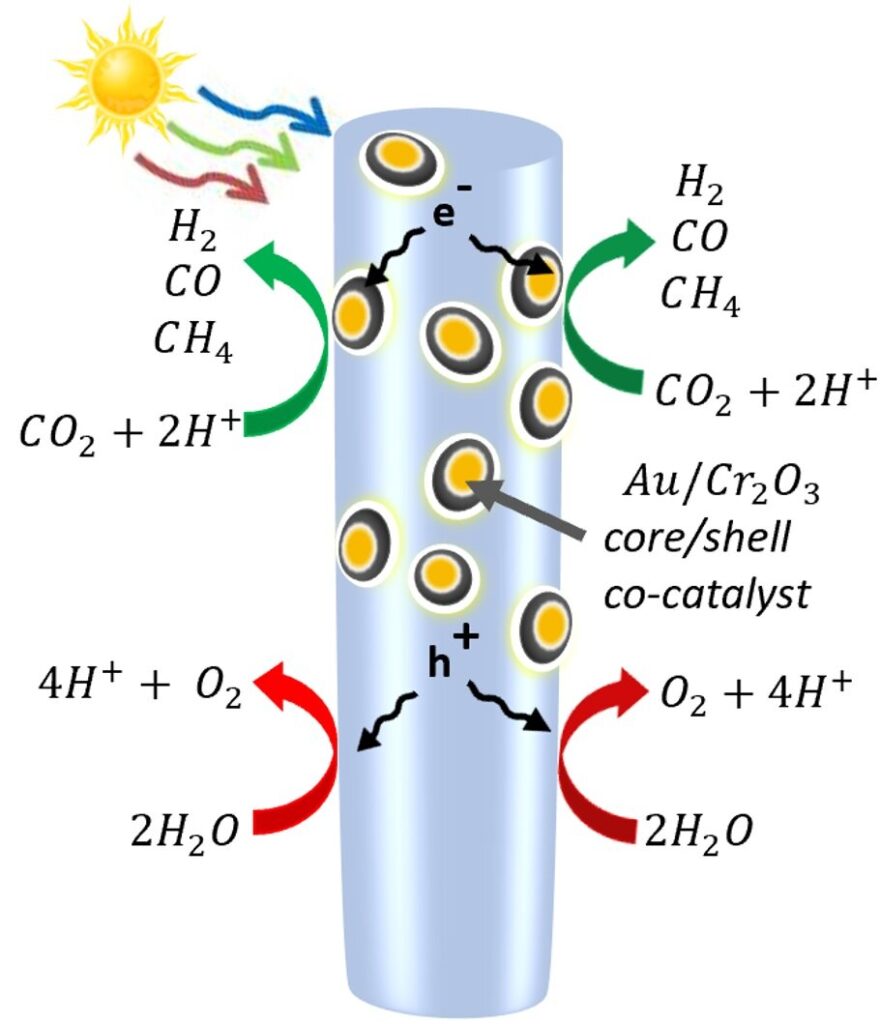
Mi’s group overcame the difficulty of splitting carbon dioxide molecules to create a process that uses only solar energy. They hit a forest of nanowires with particles. The bonds between carbon and oxygen were weakened when the gold-coated particles bent the carbon dioxide molecule.
The light energy was used to free the electrons and the holes were left behind by the nanowires. The holes split the water molecule into hydrogen and oxygen atoms. Then, at the metal catalysts, the electrons split the carbon dioxide, producing carbon monoxide and sometimes drawing in the free hydrogen to make methane. Oxygen is separated from the other gases in processes under development.
Mi’s team was able to control the amount of hydrogen and carbon monoxide produced in the reaction by changing the ratio of gold to Chromium oxide. The ratio of hydrogen to carbon monoxide affects how easy it is to make fuel or chemicals.
The synergy between gold and chromium oxide made the CO2 reduction to syngas efficient and tunable. “That was not possible with a single metal catalyst,” Mi said.
Mi’s next goal is to increase the efficiency of the device. When 10% of the light energy is converted to chemical energy, he hopes that the technology can be used for renewable energy.
More information
Roksana Tonny Rashid et al, Tunable green syngas generation from CO2 and H2O with sunlight as the only energy input, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2121174119
Latest Chemistry Research News
- Team of scientists creates first ever VX neurotoxin detector
- Scientists Discovered working principles of a promising material for fast-charging batteries
- Scientists made a step on the way to better therapies against viruses
- Scientists discovered ceramic aerogel for use in thermal insulation applications