Learning Objectives
In this article, the author has explained Avogadro’s number, molar volume, and different formulas to calculate the number of ions, particles, and molecules.
It is the number of particles (atoms, ions, or molecules) present in one mole of a substance. It is denoted by Na and its value is 6.022×1023.
One mole of different substances has different masses but same number of particles.
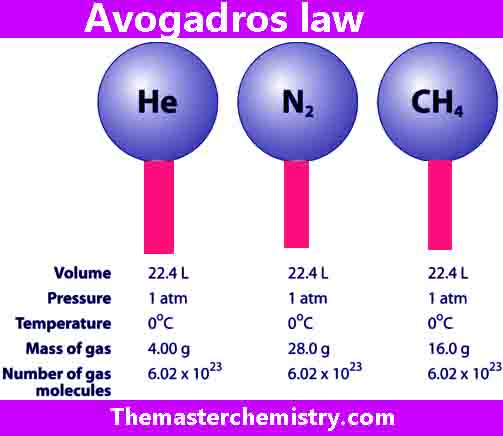
It is because individual particles of different substances have different masses, therefore, equal number of moles of different substances will also have different masses but same number of particles.
An atom of is 23 times heavier than one atom of hydrogen. Thus inn order to have same number of atoms, Na must bbe taken 23 times greater in mass than hydrogen.
Similarly, Mg atom mis twice than Carbon. Thus 10g of Mg and 5g of Carbon have same number of atoms.
Examples
Atomic mass of Na = 23.0 amu
1 mole of (23.0) g of Na = 6.022×1023 atoms
Molecular mass of H2O = 18.0 amu
1 mole of (18.0) of H2O = 6.022×1023
Formula mass of NaCl = 58.5 amu
1 mole (58.5g) of NaCl = 6.022×1023 formula units
Molar volume
Table of Contents
One mole of an ideal gas at standard temperature and pressure (STP) occupies a volume of 22.414 dm3. This volume of 22.414 dm3 is called molar volume and it is true only when the gas is ideal.
Definition of molar volume
With the help of this information, we can convert the mass of a gas at STP into its volume and vice versa.
Gas volumes are usually compared at 00C at 273 K and 1 atm. These conditions are known as Standard temperature and pressure (STP).
DEFINITION OF stp
Hence we can say that
i) 2.016g of H2= 1 mole of H2 = 6.02×1023 molecules of H2 = 22.424 dm3 of H2 at STP
ii) 16g of CH4 metahen = 6.02×1023 molecules of CH4 = 22.414 dm3 of CH4 at STP.
Here the important point to remember is that it is very interesting to know from the above date that 22.414 dm3 of each gas has a different mass but the same number of molecules with few exceptions like N2 and CO2 that have same masses, the same number of atoms and the same number of molecules in 22.414 dm3 at STP.
The reason is that the masses and the sizes of the molecules do not affect the volume. Normally it is known that in the gaseous state the distance between molecules is 300 times greater than their diameters.
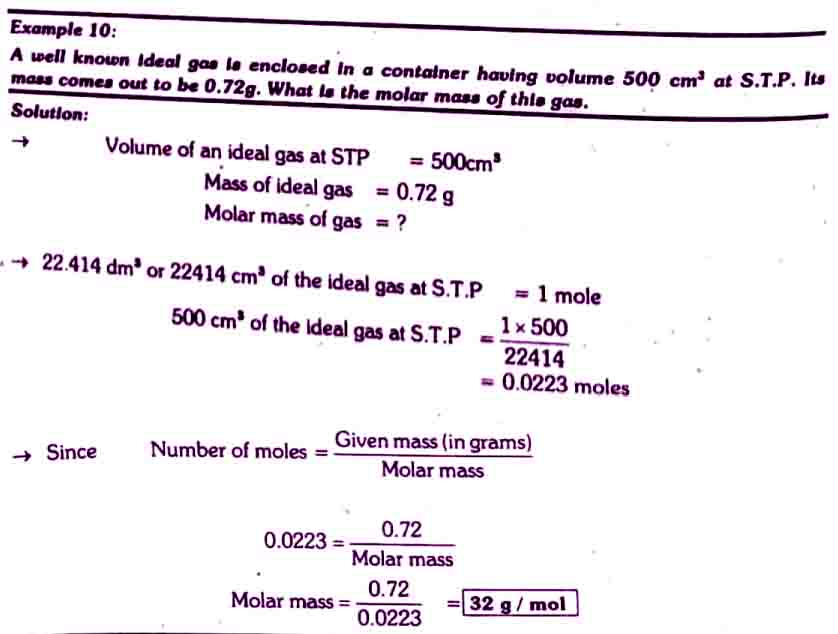
Solved example
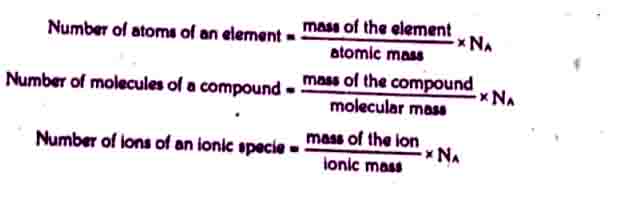
Formulas to calculate the number of particles
Number of atoms, ions and molecules can be calculated by using following formulae
FAQs
-
What does Avogadro’s number represent?
In this blog post, I will be discussing what Avogadro’s number is and why it is important. First of all, to get a better understanding of Avogadro’s number, it helps to know that there are 602 million different ways for just one person to have an arrangement of 10 pennies on the ground. This means that if you had a penny in front of you and went around picking up any other penny from the ground to add onto your pile until there were ten pennies in total, then out of those 602 million possible arrangements only one would be identical with yours! So what does this mean? It means that Avogadro’s number represents the number of atoms or molecules contained in a mole.
-
What is mole and Avogadro’s number?
Mole is a measure of the amount of substance. Avogadro’s number, on the other hand, is a measurement of how many molecules are in one mole. In order to convert between these two measurements, you have to know what type of molecule you’re dealing with. For example, if we wanted to calculate the mass in grams for 10 moles of glucose, we would divide that by 602.214 g/mol and then multiply it by 10 moles: (10 mol) * (602.214 g/mol)/(6.022 x 1023 molecules). The answer will be 6022 kg or 12000 pounds!
-
What is Avogadro’s number and how it is denoted?
A scientist named Amedeo Avogadro is most well-known for his research on gases. His work led to the development of the atomic mass unit, which is now known as “Avogadro’s number.” This post will walk you through what that means and how it is denoted.
The symbol for Avogadro’s number (N) is NA. It can be used in equations to show how many atoms are present in a given amount of gas. -
Why is Avogadro’s law important?
In chemistry, Avogadro’s law is a fundamental equation that measures the number of atoms in a given amount of substance. It is important to know how many molecules are in any given chemical reaction because it helps us understand what will happen when we add more amounts of each type of molecule together. For example, if you have 100 MLs of water and want to find out how many molecules there are in this volume, then you would divide the total volume by its molecular weight expressed in grams per mole (g/mol).