LEARNING OBJECTIVES
In this article, the author has explained air pollution, sources and effects of air pollution such as acid rain, smog, depletion of ozone, etc.
We know that any substance in the environment that has adverse effects on the following are called environmental pollutants.
- Human health
- Quality of life
- The natural functioning of the ecosystem
There are so many reasons for the rapid increase in such pollutant such as rapid growth of population, urbanization, industrialization and transportation.
All these reasons are contaminating our environment that is the top most threat of modern era.
Air pollution
Table of Contents
The mixing of harmful substances with our surroundings, particularly the atmosphere which damages the environment, human health, and quality of life is known as air pollution.
Air pollution has become a serious threat to our world.
Sources of air pollution
The sources of air pollution have been categorized into primary and secondary pollutants.
Primary pollutants
The waste products that are escaped from chimneys of industries and exhaust of automobiles.
The following gases are also included into list on primary pollutants.
- SO2
- SO3
- Oxides of nitrogen
- CO
- Hydrocarbons
- Ammonium compounds of fluorine
- Radioactive materials
Secondary pollutants
The primary pollutants which are present in the atmosphere produce secondary pollutants though various reactions.
These secondary pollutants include…
| H2SO4 | N2O |
| H2CO3 | HF |
| Peroxyacetyl-nitrate (PAN) | Ozone |
| Aldehydes | Ketones |
| Peroxybenxole |
All these compounds are toxic and their concentration in the atmosphere must be controlled.

Sources of primary pollutants
There are many air pollutants. We are going to discuss these one by one in detail.
1: Carbon Monoxide
Carbon monoxide is one of the pollutant gas.
Properties of CO
- It is a colorless, odorless, and highly poisonous gas
- It is lighter than air
- It is soluble in water
- It causes suffocation if inhaled
Its sources are
- Volcanic eruptions
- Natural gas emission
- Forest fires
- Wood and fossil fuel burning
- Oxidation of CH4 in the atmosphere
- Industries of Iron, steel, cement, brick-kilns, petroleum, paper, and pulp are the major source of carbon monoxide.
CO from fuel burning
Fuel burns in various types of transportations as
- Motor vehicles
- Railways
- Aircrafts
These sources release the 75 % of total CO in the atmosphere.
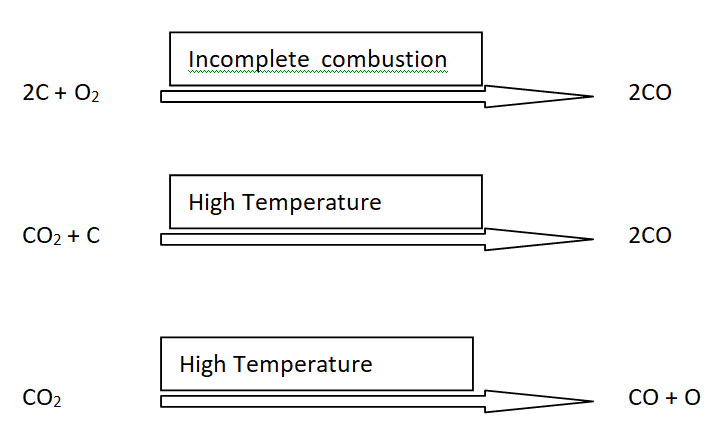
Reactions for the formation of CO
The major reasons for the formation of CO are as follows
i) Incomplete combustion of any fuel
ii) The reaction between CO2 and carbon containing material at elevated temperature like blast furnace.
iii) Dissociation of CO2 at high temperature
Poisoning effect of Carbon monoxide
When CO is inhaled it binds the blood haemoglobin more strongly than the oxygen. Due to this property it excludes oxygen from normal respiration
Oxyhaemoglobinhas a bright red colour. It decomposes and releases bonded oxygen. This way oxygen becomes more available for metabolism.
Further CO makes carboxyhaemoglobin. It is scarlet red in colour and it is much more stable.
Effects of CO on human body
When human body is exposed to high concentration of CO then, following problems may arise.
- Headache
- Fatigue
- Unconsciousness
- Eventually, death may also occur if someone gets longer exposure to CO
The effects of CO poisoning can be reversed by giving high pressure oxygen.
2: Oxides of Sulfur
Sulfur is known as eye irritating gas. The yellow color vapours of sulfur gas can causes serious breathing problem as well.
Oxides of sulfur such as SO2 and SO3 are gases with a pungent odor.
These are very irritating and suffocating.
Sources of SO2
Most of SO2 is produced by
- Volcanoes which is 67 %
- Oxidation of sulfur-containing gases produced by the decomposition of organic matter.
- Petroleum industry
- Burning of fossil fuel in power plants
- Burning of crude oil
- Combustion of coal which contains 1-9 % sulfur
Following are the reactions that produce oxides of sulfur SO2 and SO3.
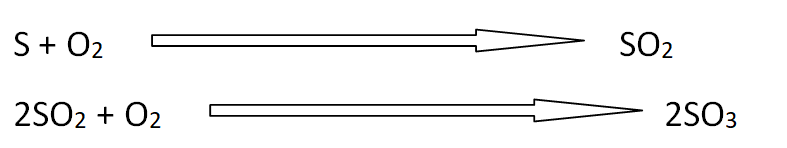
Formation of aerosols
SO2 and SO3 give various reactions in the atmosphere and these form sulfate aerosols. These aerosols are smaller than 2 micrometers. In this way, these can penetrate into the passage of lungs or human beings. This is the reason people mining sulfur from mountains covers their noses and mouth with gas mask or cloth.
When sulphates and aerosols are inhaled these cause acute respiratory troubles especially among the old people.
SO2 presents another serious threat of acid deposition in the atmosphere.
3: Oxides of nitrogen
Nitrogen gives five oxides. Among these five NO and NO2 are frequently produced in the atmosphere. These are responsible for air pollution and represented as NOx.
Sources of oxides of nitrogen
The main sources of oxides of nitrogen are as follows:
- Combustion of coal
- Combustion of oil
- Combustion of natural gas
Atmosphere and nitrogen present in the fossil fuels re the source of nitrogen to form oxides. When oil is burnt in internal combustion engines of vehicles, then NO is produced.
NO2 is produced when NO reacts with Oxygen.
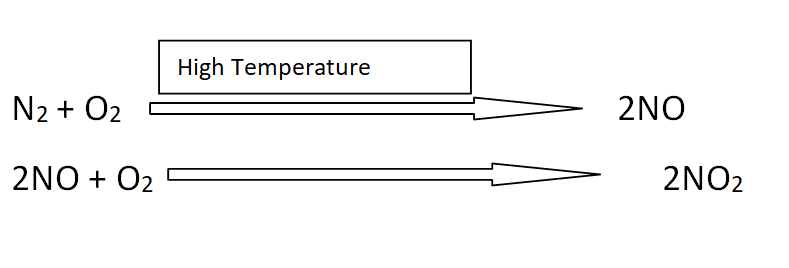
How oxides of nitrogen reach the earth?
The major part of nitrogen is present in our atmosphere and it forms oxides through various reactions.
The question arises of how these oxides of nitrogen reach the earth. Actually, NO and NO2 can exist in the atmosphere just for 3- 4 days. Due to sunlight present in the atmosphere, a photochemical reaction converts oxides of nitrogen into nitric acid.
This nitric acid further reacts with other substances to form nitrates. Finally, these nitrates reach the earth along with dust rainfall.
4: Hydrocarbons
Hydrocarbons are the compounds of carbon and hydrogen. The most important hydrocarbons which are used in our daily life are methane and petroleum products. Therefore, we can say that we are surrounded by hydrocarbons in today’s rapidly growing world.
Natural gas
The average residence time of methane is about 3-7 years in the atmosphere. Natural gas is obtained from the following sources
- A large number of hydrocarbons are emitted by different trees and plants in the atmosphere.
- Paddy fields contribute a major amount of methane to the atmosphere
- Anaerobic decomposition of organic matter by bacteria in water produces methane.
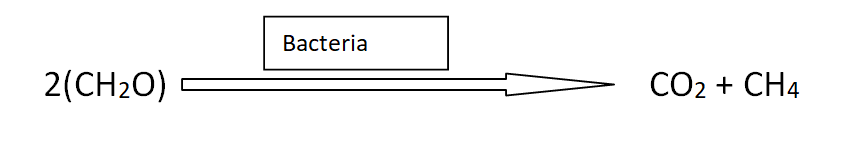
4: Domestic animals also contribute to natural gas.
Although the major source of hydrocarbon is petroleum. Other hydrocarbons are obtained through fractional distillation of petroleum.
However in addition some other sources also produce different Hydrocarbons in the atmosphere as a result of human activities.
- Automobiles
- Coal
- Wood
- Solvent evaporators
- Incinerators
Effects of air pollution on the environment
Air pollution effects the environment in many ways. Let us discuss these one by one in detail.
1: Acid rain
Concept of acid rain was presented by Augus Smith in great Britain during 17th century.
This phenomenon became important as environmental threat in 1950s.
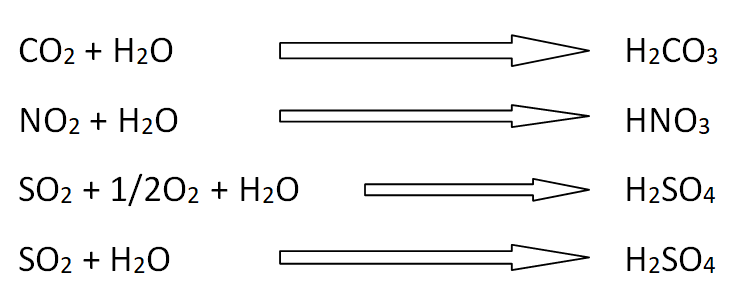
The oxides of carbon, nitrogen and sulfur are responsible of acid rain.
Due to the presence of CO2 in the atmosphere, the natural rain itself forms the carbonic acids.
NO2 in the atmosphere combines with water to give HNO3.
SO2 and SO3 react with water to give H2SO4.
Acid rain due to HCl from Volcanic eruption
In some countries of the world HCL gas is produced by the volcanic eruption. It is a major reason for the temporary acid rain.
Acid rain pollution
Oxides of nitrogen and sulfur emitted by the different sources are converted into acid rain by reacting with rain water.
Effects of acid rain
- Acid rain makes the soil rivers and lakes slightly acidic
- Soils and rocks also become acidic in nature
- The acidic nature of soil and rocks causes leaching of the metals like Al, Hg, Pb, and ca. These metals are discharged into water bodies and present a threat to aquatic life.
- These heavy metals enter into fishes and fishes are eaten by humans. Thus it affects humans indirectly as well.
- The higher concentrations of Aluminum is harmful to fishes because it clogs the gills of fishes leading towards suffocation.
- When soil is acidified by acid rain then it can leach the nutrients. This will damage the leaves and plants and the growth of the forest.
The acid rain also damages the domestic parameters such as
- Steel
- Plastic
- Paint
- Cement
- Constructional projects
- Marbles and limestone buildings
- Statues and monuments made with stones
2: Smog
Smog is a combination of smoke and fog.
Smog has become a very fetal threat to life on the earth. Due to rapid increase in burning of fossil fuels it has become more dangerous in urban areas.
Types of smog
Reducing smog
If it contains high amount of SO2, then it is reducing in nature and is known as reducing smog.
Photochemical smog
This smog is consisted of high concentration of oxidants like ozone. It is also called oxidizing smog.
Properties of photochemical smog
- It is yellowish band brownish-grey haze with an unpleasant odor
- The main reactants of photochemical smog are NO and unburnt hydrocarbons.
- The yellowish color of smog is due to the presence of NO2
Sources of smog
Combustion of coal
Photochemical smog is due to chemical reactions of pollutants in air
How smog is formed?
During winter season when the smoke is combined with fog it form smog. Basically in smoke there are some pollutants that accelerate the smog formations.
Conditions for the formation of smog
- There should be sufficient NO, hydrocarbons, and volatile organic compounds( VOCs). These substances are emitted by vehicles in urban areas.
- At the time of chemical reaction, sunlight is required to make the reaction fast.
- The movement of the air must be minimized so the reactions are not disturbed.
- The overall result of photochemical smog in the afternoon is the build-up of oxidizing agents.
Following are some important oxidizing agents in this respect.
- H2O2
- HNO3
- Peroxyacetyl-nitrate
- Ozone
Peroxyacetyl-nitrate causes irritation in eyes and is also toxic to the plants.
3: Depletion of Ozone layer
Ozone is an allotrope of oxygen. It is produced in tropical regions by the photochemical reactions of oxygen. Then it is transported to polar regions. It surrounds the globe at a distance of 25-28 km.
Properties of ozone
- Ozone is a gas at room temperature
- It has a low boiling point
- It has a low concentration throughout the atmosphere.
How ozone concentration is measured?
The amount of ozone in the atmosphere is expressed in Dobson unit which is represented as DU. The normal amount of overhead ozone is about 350 DU.
Purpose of ozone
Ozone layers helps in many ways protecting our planet. It filters most of the harmful UV rays present in the sunlight. In other words its protects the earth from UV and many other radiations that causes many physiological and climatic threats.
If the ozone layer becomes thin the life on earth would be threatened. In 1980s a large hole in the ozone layer was discovered. This hole was detected in the Antarctic region. Since that time there is a major environmental crisis.
Ozone as a pollutant
Ozonen acts as a pollutant and causes various health problems as well.
- It damages eyes
- Aggravates asthma
- Decrease the elasticity of lung tissues
- Increased coughing
- Creates chest discomfort
- Harms to plants and other materials
- Attacks the rubber material
- Reduces durability and appearance of paint
- Causes fabric dyes to fade
Variation of ozone on earth
The variation of ozone varies from region to region.
- The amount of ozone is less in the regions close to the equator.
- The average ozone amount in the stratosphere of the tropical regions is about 250 DU.
- In subpolar regions, its average concentration is 450 DU.
- The concentration of ozone changes with seasons.
- It is at the highest level in the early spring. In February-March and is the lowest in September-November.
Change in thickness of ozone layer
The thickness of the ozone layer has been decreasing drastically over Antarctica during the spring times. This decrease of thickness started since the mid 1970s.
By the middle of 1980s there happened a 50 % depletion of the total overhead amount at some altitudes over Antarctica.
Ozone hole
The region in which the ozone depletes substantially every year from September to November is now called the ozone hole.
Ozone and stratosphere
Different chemical reactions are responsible for ozone depletion in the stratosphere. This is not only happening on Antarctica, but it is happening worldwide.
The ozone layer exists in the atmosphere in the stratosphere. It is approximately at a distance of 15-40 km altitudes. It is just above the troposphere which extends to an altitude of 10-15 km from the earth.
The temperature after the troposphere decrease with increasing altitude from 15-56 oC. The reason is that the air which is near the earth is heated by radiations remitted from the earth.
The temperature in the stratosphere increases with the increase of altitude from -56 — -2oC.
Ozone protects the earth from UV radiations
Ozone is the main chemical specie present in the stratosphere. It absorbs the UV radiations and increases the temperature in the upper part of ozone layer. The range of UV light is from 50-400 nm. it is more energetic than visible light and is very harmful.
Therefore, a screen layer of ozone has been provided by the nature to protect us in the stratosphere.
Causes of ozone depletion
Chlorofluorocarbons are very important compounds which are produced on industrial and domestic scale in the world. These compounds are used as refrigerants in air conditioning.
These are also used as aerosol sprays. These are inert in the troposphere. However, these slowly diffuse to the stratosphere. In the stratosphere, these are exposed to ultraviolet radiations. These radiations produce chloride-free radicals. These chloride-free radicals react with ozone and are converted into oxygen.
The following reactions show ozone depletion.
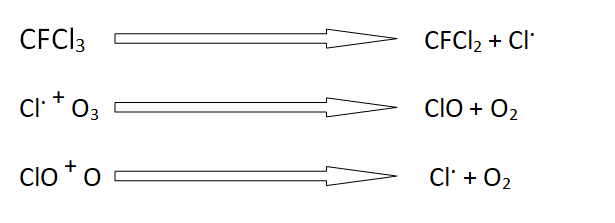
A single chloride free radical can destroy up to 100,000 ozone molecules.
Further readings