Learning objectives
In this article, the author has explained what is cracking, the cracking of petroleum, types of cracking, examples of cracking, and applications of cracking.
Cracking of petroleum
Table of Contents
Definition of cracking
There are two possible definitions of cracking.
The thermal decomposition of alkanes in the absence of air is called cracking.
The process of decomposition of less volatile higher hydrocarbons into more volatile lower hydrocarbons with the application of heat and catalyst is called cracking.
Definition of cracking of petroleum
Watch the video to better understand the cracking of petroleum
It is also known as pyrolysis.
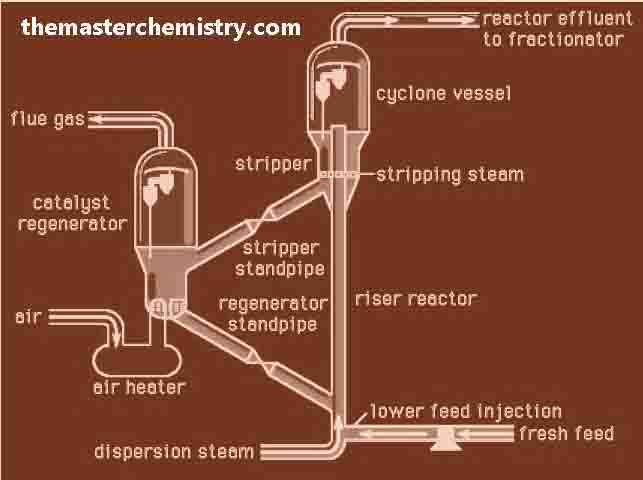
Importance of cracking
The fractional distillation of petroleum only gives us 20 % gasoline. This is a very small fraction of the total gasoline used by the world. The higher demand of the present civilization can be carried out by cracking of higher alkanes.
These higher alkanes mostly consist of kerosene oil. In fact, approximately 50% of gasoline is now prepared through this method.
Examples of cracking
In order to illustrate the process of cracking and pyrolysis, let us try to understand the process by cracking methane.
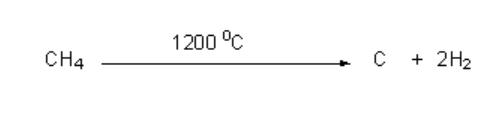
In this reaction carbon black which is produced is used as a filler in the manufacture of tires and hydrogen produced is sold in the other industries.
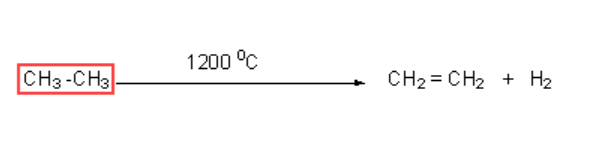
This reaction provides one of the ways to get ethane from ethane.
Similarly, the pyrolysis of propane and butane gives us a variety of products.
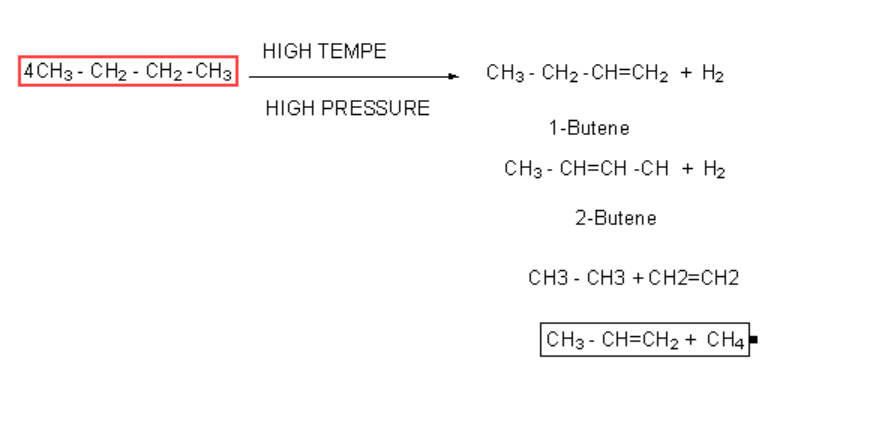
Greater the number of carbon and hydrogen atoms in alkane greater the variety of products. A higher hydrocarbon C16H34 is craked according to the following reaction.
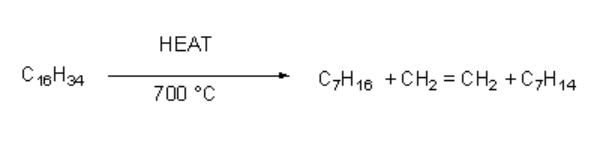
Nautre of the products in cracking
The nature of products from the given compound depend upon the following factors.
- Structure of hydrocarbons
- Temperature and pressure used in cracking
- Nature of catalyst used in cracking
Types of cracking
Cracking is of three types.
- Thermal cracking
- Catalytical cracking
- Steam cracking
Thermal cracking
This crackcing is carried out by the application of heat and pressure. The products after cracking are passed through the fractionating tower. In this way the gasoline can be separated from the fractions. Thermal cracking is done both in liquid and vapor phase.
In the liquid phase, the craking temperature of 470-530 °C is maintained along with the pressure of 7-70 atmosphere.
In a vapor-phase cracking, the temperature is 600 °C and the pressure is 3.5-10.5 atmosphere.
Also read:
Catalytical cracking
This cracking is done in the presence of catalyst. The temperature and pressure are comparatively low.
Catalysts used for cracking
Typical catalyst used for this purpose is the mixture of silica (SiO2) and alumina (Al2O3). The mixture of these two substances give us aluminum silicates. Catalytical cracking produces gasoline of higher octane number. In this way the gasoline of better quality is obtained.
Steam cracking
Higher hydrocarbons are converted to vapors and mixed with steam. This mixture is heated for a short time to about 900 °C. It is then cooled rapidly. This process is suitable for obtaining lower unsaturated hydrocarbons.
Applications of cracking
Following are some important applications of the cracking..
Preparation of gasoline ( Petrol for cars)
More then 50 % petrol is produced by cracking. It gives better quality of petrol in term of anti-knocking property.
Preparation of oil gas
Oil gas is a mixture of methane, ethane, propane, butane, and very small fractions of other hydrocarbons along with hydrogen.
Oil gas is obtained by the cracking kerosene oil by dropping it over red hot iron retort.
Preparation of petrol gas
Petrol gas is prepared by the cracking of petrol by passing it through electrically heated coils.
Polymerization
Simple alkene which are produced in the pyrolysis are used to prepare polymers. These polymers can be the polymers of petrol.
The simple hydrocarbons like ehtne, propene, butane and benzene are used for the manufacturing of following.
- Drugs
- Plastic
- Detergents
- Synthetic fiber
- Fertilizers
- Weed killer
- Chemicals like ethanol, phenols, and acetone