LEARNING OBJECTIVES
In this article, the author has explained 7 Difference Between Molecule and compounds.
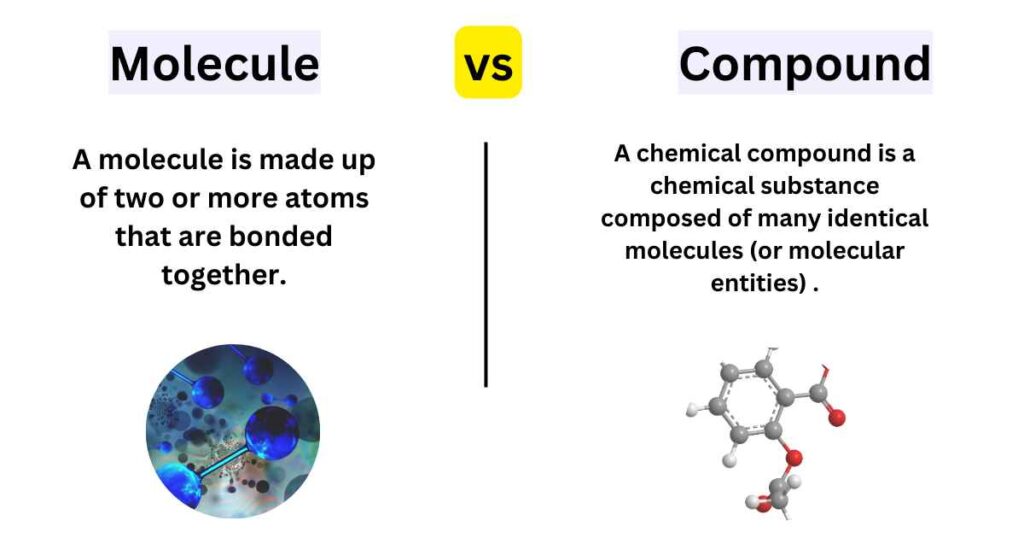
The main difference between molecules and compounds is the difference in their composition. A molecules consist of two or more bonded atoms, while compounds are formed by the combination of different elements.
In chemistry, a molecule is a substance consisting of two or more atoms that are chemically combined in definite proportions by weight. A compound, on the other hand, is composed of two or more chemical elements and may also contain different types of molecules. In this blog post we will explore the difference between these two terms!
Read more such articles
Molecule VS Compound
- The main difference Between a Molecule and a Compound is that a molecule is made up of two or more atoms that are bonded together, while a compound has molecules. Molecules and compounds can both be broken down into their component parts by chemical analysis.
- the second Difference Between Molecule and Compound is that Compounds have a definite chemical composition and Molecules do not.
- This Difference Between Molecule and Compound means that you can identify the number of atoms present in a compound, while it is impossible to determine how many molecules are within a substance when studying chemistry.
- Fourth Difference Between Molecule and Compound is that, Molecule is a small particle of an element or compound that has all the properties (chemical and physical) of its parent substance. On the other hand, Compound is neutral combination made by two or more chemical elements which can be looked at as ‘molecules’.
- The fifth Difference Between Molecule and Compound is that a molecule has at least one element, whereas a compound contains two or more elements.
- Sixth Difference between Molecule and Compound is that a compound is capable of reacting with other compounds to form new substances, while molecules are not.
- Seventh Difference Between Molecule and Compound is the number of components in each either one atom or many atoms as part of a single unit as well as it’s chemical formula.
Here’s a table summarizing the differences between Molecule and Compound:
| Difference | Molecule | Compound |
|---|---|---|
| Composition | Made up of two or more atoms bonded together | Has molecules |
| Chemical Composition | Does not have a definite chemical composition | Has a definite chemical composition |
| Identification | Number of molecules cannot be determined | Number of atoms present can be identified in a compound |
| Particle Size | Small particle of an element or compound with properties of its parent substance | Neutral combination made by two or more chemical elements |
| Number of Elements | Contains at least one element | Contains two or more elements |
| Reactivity | Molecules do not react with other compounds to form new substances | Compounds are capable of reacting with other compounds to form new substances |
| Components | Can have one atom or many atoms as part of a single unit | Can have multiple atoms as part of a single unit |
| Chemical Formula | No specific chemical formula | Can be represented by a chemical formula |