LEARNING OBJECTIVES
In this article, the author has explained differences between rust and corrosion.
The main difference between rust and corrosion is the difference in appearance and formation.
In this blog post, we will discuss some of the key differences between these two types of metal degradation so that you can spot them faster in your home or business!
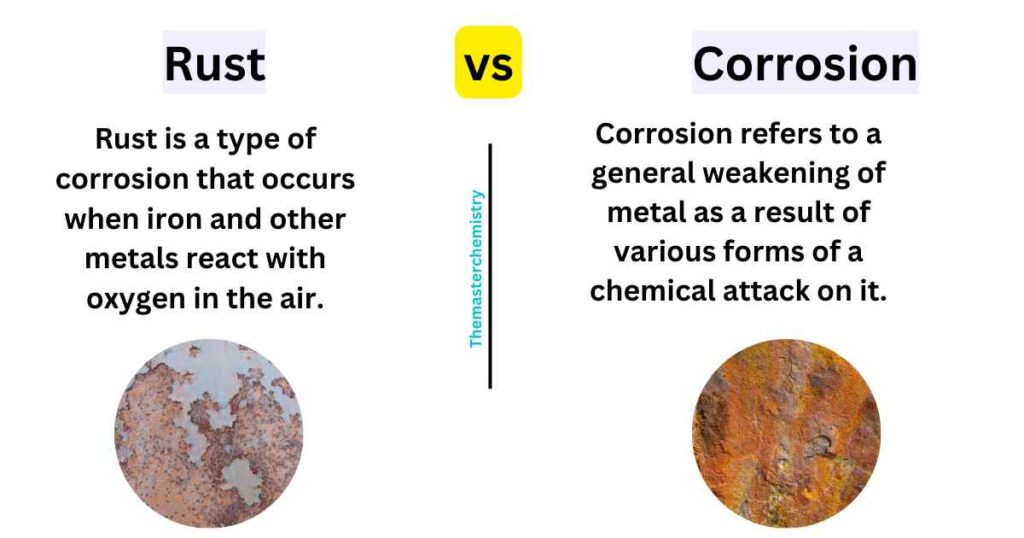
Rust is a type of corrosion that occurs when iron and other metals react with oxygen in the air. This reaction produces carbon dioxide, water, and ferric oxide. The ferric oxide gives rust its reddish-brown color.
On the other hand, corrosion refers to a general weakening of metal as a result of various forms of a chemical attack on it. Corrosion can occur without any contact with air or water because there are many corrosive chemicals in everyday life such as acids and alkalis which can cause severe damage to unprotected metal surfaces.
Rust VS Corrosion
1: The main difference between rust and corrosion is that rust is typically red or brown in color, whereas corrosion tends to be lighter and very thin.
2: The second first difference between rust and corrosion is that Rust gets its coloring from the metal that it forms on top of while corrosion appears as a flaky layer similar to paint peeling off of an old wall.
3: The third first difference between rust and corrosion is that rust generally forms on top of the metal it’s acting upon, while corrosion can appear underneath.
4: The fourth difference between rust and corrosion is that Rust often appears in multiple layers while Corrosion tends to be singular in nature.
5: The fifth difference between rust and corrosion is that both are caused by different processes – Rust being a result of oxygen reacting with iron atoms, whereas corrosion comes from moisture degrading metal molecules into ions which then form an oxide layer or hydroxide salts. This process will continue until no more oxidation occurs but without any actual damage to the metal itself.
6: The sixth first difference between rust and corrosion is that corrosion is a chemical reaction. Corrosion occurs when the metal reacts with oxygen and water, producing rust as a by-product of that process.
7: Corrosion can be accelerated through exposure to toxic chemicals or salt in seawater which you should take into consideration if your boat will have to survive those conditions regularly.
In fact, many boats are made from aluminum because it does not corrode as quickly as steel, but it still needs regular maintenance to prevent surface damage due to oxidation reactions occurring on their surface so much more slowly than they do for iron or other metals used in shipbuilding materials.
If left untreated, even small scratches caused by hail stones during storms can become major problems down the road requiring expensive restorative work like sanding, priming, and repainting.
7: The seventh first difference between rust and corrosion is that rust is an element and corrosion occurs when the metal itself has been compromised.
When steel or iron is exposed to salt water, chemicals in seawater begin to corrode the material from within. This happens because of a chemical reaction between oxygen molecules dissolved in water called oxidation-reduction reactions.
Rusting can also occur if there isn’t enough humidity to keep your metals moist, but it will take longer without moisture present so that’s why you should always wipe down all surfaces after being outside during the winter months. And for this same reason, humid weather encourages both forms of corrosion! gaining them back (oxidation).
It’s possible for other elements such as chromium to oxidize too – this chemical reaction creates a rust color! Chromium reacts differently than iron though because it doesn’t lose any particles during oxidation; instead, its hydrated form gains hydrogen gas from water to create an acidic solution.
Here’s a table summarizing the differences between rust and corrosion:
| Difference | Rust | Corrosion |
|---|---|---|
| 1 | Typically red or brown in color | Lighter and very thin |
| 2 | Coloring comes from the metal it forms on top of | Appears as a flaky layer similar to peeling paint |
| 3 | Generally forms on top of the metal | Can appear underneath the metal |
| 4 | Often appears in multiple layers | Tends to be singular in nature |
| 5 | Result of oxygen reacting with iron atoms | Moisture degrades metal molecules, forming oxide layer/salts |
| 6 | Rust is a by-product of the corrosion process | Corrosion is a chemical reaction |
| 7 | Rust is an element | Corrosion occurs when the metal itself has been compromised |
| 8 | Can be accelerated by exposure to toxic chemicals or saltwater | N/A |
| 9 | Common in steel or iron | Can occur in various metals and materials |
| 10 | Requires regular maintenance to prevent further damage | Requires maintenance to prevent surface damage |
Also read
Difference between oils and fats
Difference Between Evaporation and Boiling
Difference between Cell and Battery
Differences between Vapor and Gas