LEARNING OBJECTIVES
In this article, you will learn the basic differences between orbit and orbital
Watch the video lecture for a better understanding of the topic
This post is for all the people who are wondering what the difference between Orbit and Orbitals. I am going to do my best to explain what Difference between Orbit and Orbitals.
There are two terms in chemistry that you need to know about orbitals: orbit and orbital. An orbital is an area on an atom that has electrons that can be lost or gained by chemical reactions, whereas orbits are paths of movement around a central object.
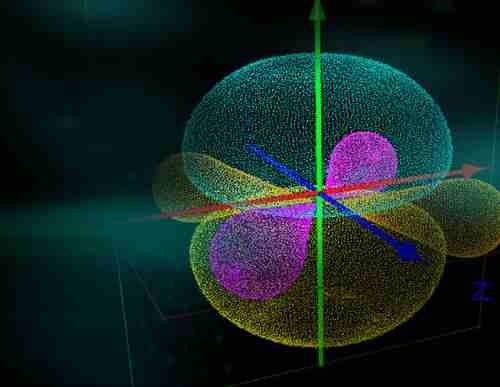
Why it is important to know the difference between orbit and orbital in chemistry?
It is important to know the difference between orbit and orbital as chemistry is a multi-faceted subject that is constantly evolving and the language used to describe it will change as well. The difference between orbit and orbital is simply that an atom’s electron “orbits” around the nucleus. This movement defines them as either s or p electrons, which determines what type of chemical they will be involved in.
9 Difference between orbit and orbitals
- The main difference between orbits and orbitals is that orbitals are regions where an electron is most likely and orbits do not have a high possibility of having their electron present in them.
- The second difference between orbits and orbitals is that there are different types of orbital shapes for every atom depending on its position in the periodic table. There are four types of orbitals, the s orbital, p orbital, d orbital and f orbital.
- The third difference between orbits and orbitals is that orbitals are not visible, whereas orbits are.
- The fourth Difference between orbit and orbitals is that electrons are present in orbitals as opposed to orbits.
- The fifth Difference between orbit and orbitals is that electrons orbitals are different from each other. In general, the number of s and p orbitals equals the number of elements in a group.
- The sixth Difference between orbit and orbitals is that orbitals are a three-dimensional shapes of electron density while orbitals only exist in two dimensions.
- Orbitals can be defined as mathematical equations that define the amount and location of electrons surrounding an atom or molecule, whereas orbit is where they’re likely to be found at any given time. Orbitals have been described by scientists as “probability clouds” because they are constantly changing.
- The seventh Difference between orbit and orbitals is that the shapes of electron density in an orbital can be spherical, dumbbell-shaped or disk-shaped. Orbitals have also been described as ovals, mushrooms and doughnuts due to their varying shapes! However, orbitals are only two-dimensional.
- The eighth Difference between orbit and orbitals is that atoms exist in three different states, or energy levels: ground state, excited state and ionized state. Orbitals can be defined as mathematical equations which define the amount of electrons surrounding an atom or molecule whereas orbital refers to where they’re likely to be found at any given time.
- the ninth Difference between orbit and orbitals is the amount of energy required to remove an electron.
