written by Adeel Abbas
The key difference between physical and chemical changes is the difference in the rearrangement of particles. In a physical change, there is no rearrangement of particles, but in a chemical change, particles are rearranged, resulting in the formation of new products.
Physical and chemical change are both forms of change that are caused by interactions within the system. Differences between physical and chemical changes are important to understand because they can have different effects on systems.
Read more such articles
Physical vs Chemical change
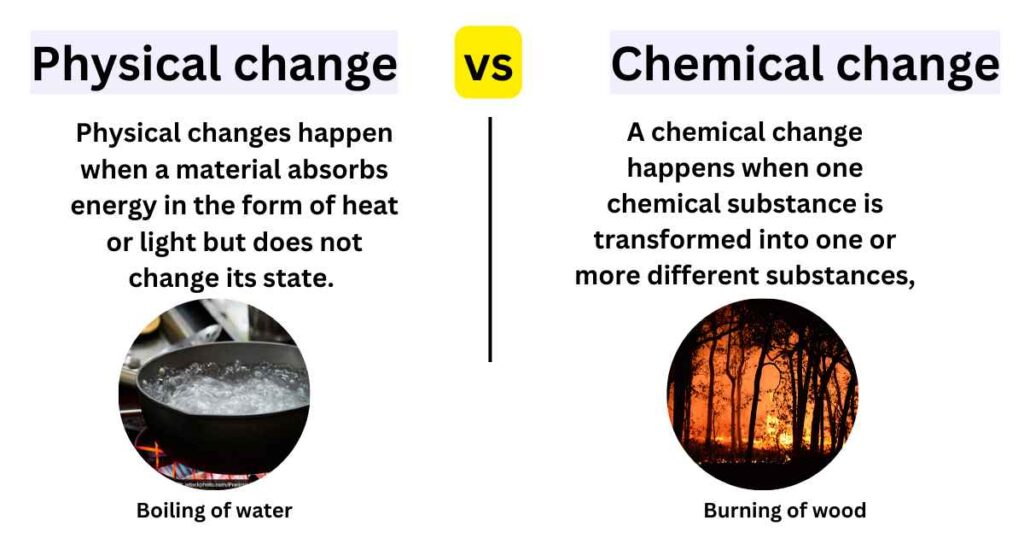
- Physical change is a process that does not involve the rearrangement of particles, while chemical change is a process that involves the rearrangement of particles.
- Physical change is typically a result of an external stimulus, while chemical change is initiated by a chemical reaction.
- Examples of physical change include melting ice, evaporating water, and freezing cold air, while examples of chemical change include burning wood and digestion.
- Physical change absorbs energy in the form of heat or light, whereas chemical change requires an input of energy (activation energy).
- Physical change is reversible, meaning the original state can be restored, while chemical change is usually irreversible.
- Physical change does not produce new substances or alter the fundamental composition of the substance, whereas chemical change results in the formation of new products with a different composition.
- Physical change does not require a chemical equation to describe the process, while chemical change is often represented using a chemical equation.
- Physical change can be explained by changes in internal kinetic energy, while chemical change involves changes in potential chemical bond energy and the movement of particles.
- Physical change can occur without an input of activation energy, whereas chemical change requires an activation energy barrier for the reaction to occur.
Here’s a table summarizing the differences between physical changes and chemical changes:
| Aspect | Physical Change | Chemical Change |
|---|---|---|
| Rearrangement of Particles | No rearrangement of particles, only an external stimulus | Rearrangement of particles occurs |
| Examples | Melting ice, evaporating water, freezing cold air | Burning wood to produce ash and smoke, digestion |
| Energy Absorption | Absorbs energy in the form of heat or light | Requires an input of energy |
| Reversibility | Reversible | Irreversible |
| Formation of New Products | Does not produce new products | New products are formed |
| Nature of Change | No change in the fundamental composition of the substance | Changes the fundamental composition of the substance |
| Representation | No chemical equation required | Can be represented using a chemical equation |
| Activation Energy | No activation energy required, only external stimulus is needed | Requires an activation energy barrier for the reaction to occur |
| Kinetic Energy Change | Changes in internal kinetic energy due to external influence | Changes in potential chemical bond energy and movement of particles |