LEARNING OBJECTIVES
In this article, the author has explained difference between vapor and gas in detail.
Many people are not aware of the differences between vapor and gas. Vapor is a substance that has been heated to its boiling point, but below its critical temperature, which causes it to evaporate into a gas.
Gas is any matter that may be in either liquid or solid form at room temperature and atmospheric pressure. Differences between these substances include their density, solubility, boiling points, and freezing points.
Differences between Vapor and Gas
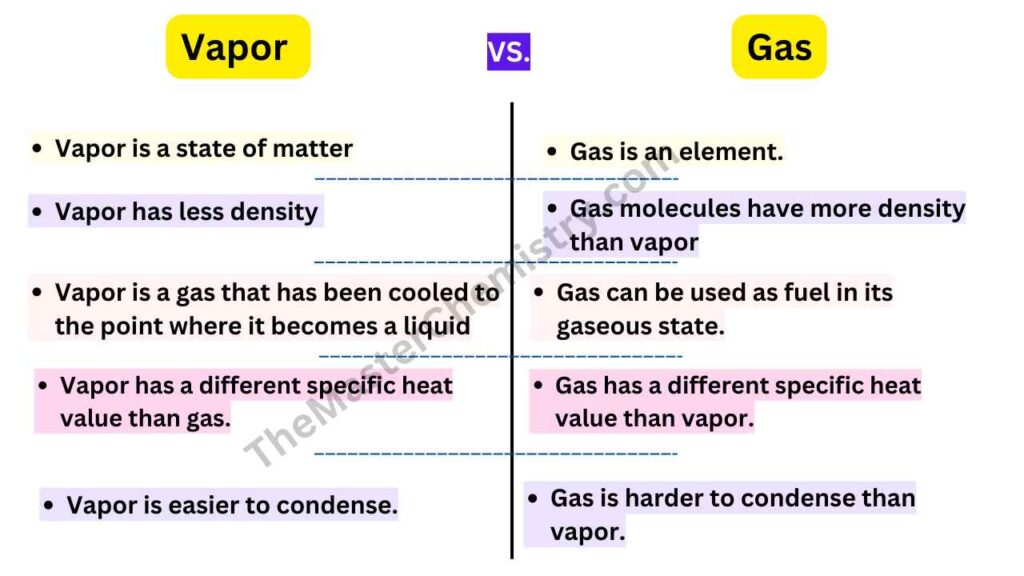
- The main difference between Vapor and Gas is that Vapor is a state of matter and Gas is an element.
- The second Difference between Vapor and Gas is that vapor has less density than gas molecules, which means it will rise above its liquid counterpart.
- The third difference between Vapor and Gas is that they both have different boiling points; The temperature at which one changes from liquid to gas (vapor) is known as the boiling point.
- The fourth Difference between Vapor and Gas is that vapor is a gas that has been cooled to the point where it becomes a liquid, and can then be used as fuel.
- The fifth Difference between Vapor and Gas is that they both have different specific heat values. The amount of energy required to change one gram of a substance’s temperature by one degree Celsius or Kelvin.
- The sixth Difference between Vapor and Gas is that Vapor is easier to condense. There are many ways Vapor can be condensed, which makes it easy for scientists to take the Vapor and turn it back into a liquid or solid.
Related articles
- 6 differences between CNG and LPG By Adeel Abbas
- 9 Differences between Thermoplastic and thermosetting plastic
- Difference between compound and mixture
- 7 Differences between baking soda and baking powder
- 7 Differences Between Vaporization And Evaporation
- A Clarification of Difference between Orbit and Orbitals