LEARNING OBJECTIVES
In this article, the author has explained what is atomic structure, discovery of electron, proton and neutron.
Atomic structure
Table of Contents
In 1808, an English Chemist John Dalton gave Dalton’s atomic theory. According to this theory matter is composed of very small particles, which are invisible .These particles are called atoms.
But experiments done by brilliant research workers proved that atom is divisible.
Different scientists in this field are as follows:
1. J.J Thomson
2. Rutherford
3. Bohr
4. Sommerfeld
5. Moseley
6. Chadwich
What are Sub-atomic particles of atoms
The atom is consisted of several particles called subatomic particles. Some of these are electron, protons, neutron, positron, neutrino, and meson etc. Out of these particles electron, proton and neutron are very important. Hence these are called fundamental particles.
Let us discuss in detail the discovery and properties of electron, proton and neutron.
Related: Evidence of Atom
DISCOVERY OF ELECTRON (CATHODE RAYS)
IN 1876, W.CROOKS conducted a series of experiments. He used a discharge tube filled with a gas fitted with two electrons.
He observed that there was no flow of current at ordinary pressure of the gas. However, when the pressure inside the tube was reduce and high voltage of 5,000-10,000 volts was applied the electron circuit was completed. A uniform glow inside the tube was produced.
The colour of the glow depend upon the nature of the gas within the tube. When the pressure of the gas was reduce to about 0.01 torr, the original glow disappeared. Some invisible rays were produced. These rays produced a fluorescence on the glass wall opposite to the cathode. These rays were called cathode rays.
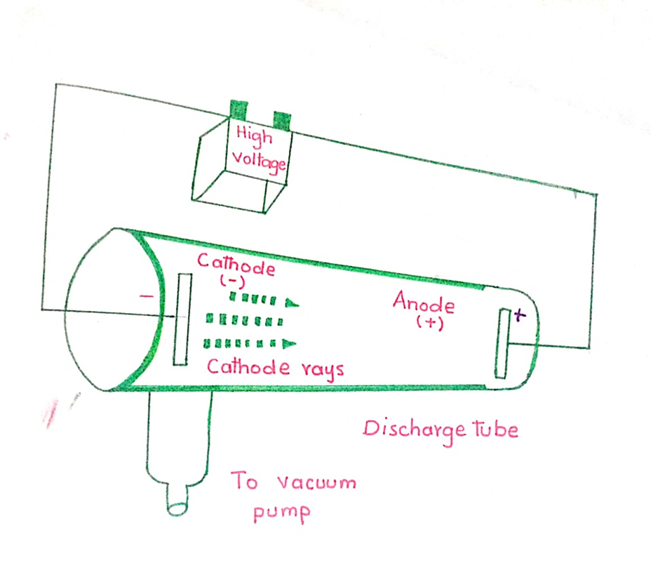
PROPERTIES OF CATHODE RAYS
1. Way of travelling
These rays travel perpendicular to the surface of the cathode and its seems that they originate from the cathode.
2. Effect of electric field
These rays are deflected towards the positive plate, when passed through the electrical field. Its means that they are negatively charged.
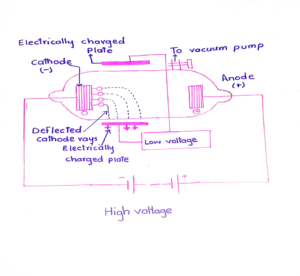
3. Effect of magnetic field
When cathode rays are passed through the magnetic field they bend perpendicular to the joining lines of the two poles.
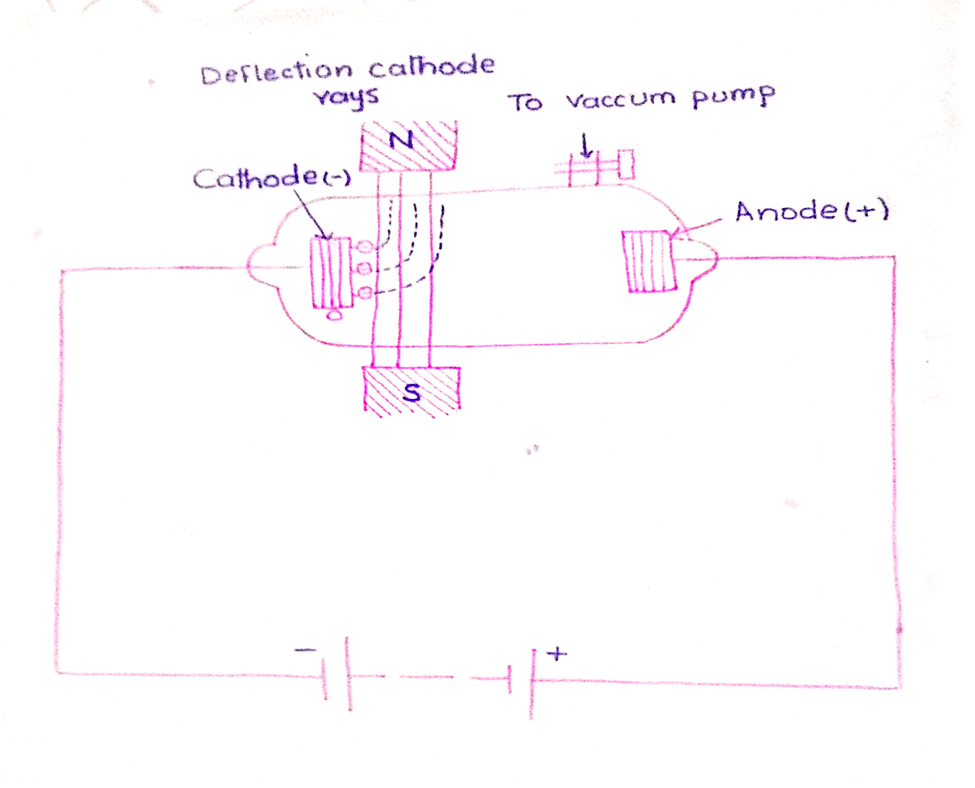
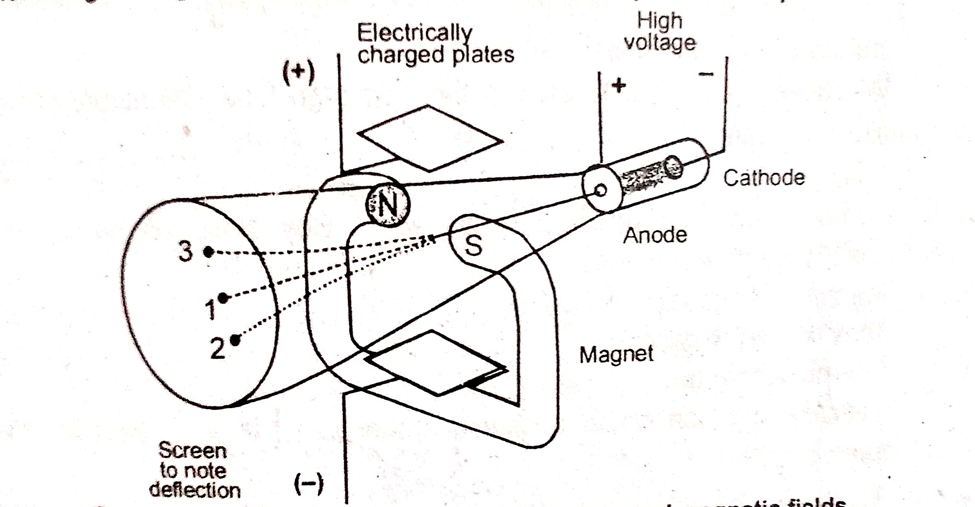
The simultaneous application of electric and magnetic field can be explained from the following arrangement, which was demonstrated by J.J Thomson.
4. Shadow of opaque object
They produce a sharp shadow if an opaque object is placed in their path.
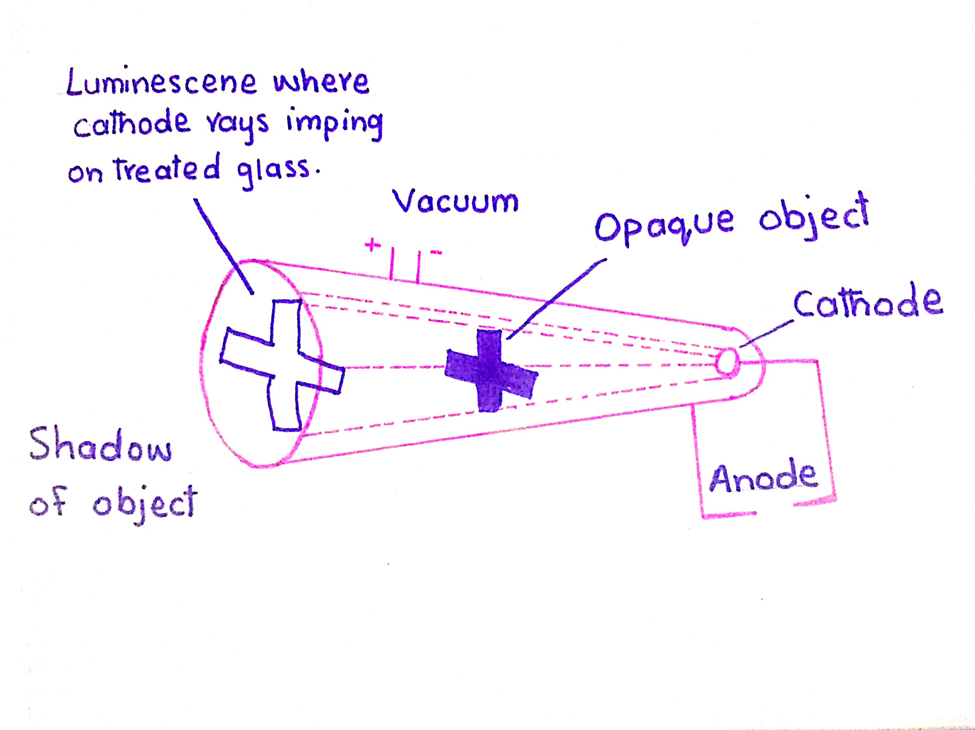
5. Fluorescence
They produce fluorescence on the walls of the glass tube opposite to that of the cathode.
6. Mechanical motion of paddle wheel
These produce a magnetic motion in a paddle wheel, which is placed in their path.
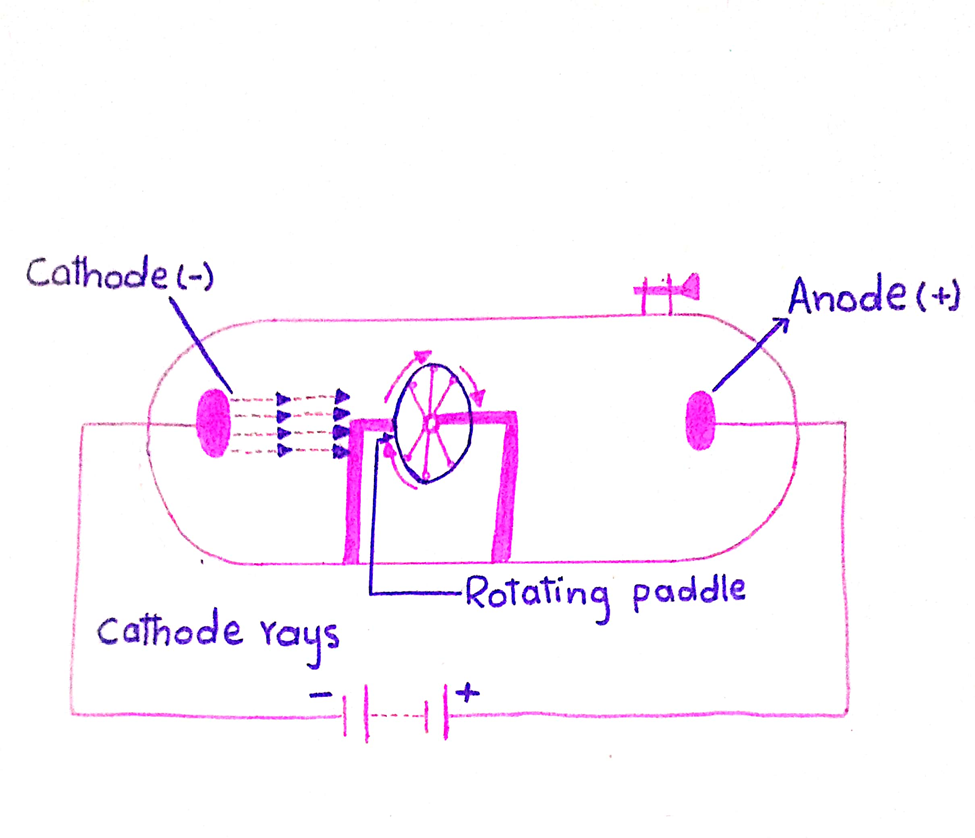
7. Kinetic energy
These rays raise the temperature of the object, which is placed in their path. It indicates that they possess kinetic energy.
8. Nature of rays and gas
Whichever gas is used in the discharge tube the nature of the cathode rays remain the same.
9. X-Rays
Cathode rays produce x-rays when they strike an anode, particularly with large atomic mass.
10. Ionization of gas
They can ionize gasses.
11. Chemical change
Cathode rays can cause a chemical change because they have reducing effect
12. e/m values
The e/m value of cathode rays is just equal to that of electron. It means that cathode rays are nothing but electrons.
Conclusion
1. Atom is breakable.
2. Electrons is essential part of matter
Reasons for cathode rays formation
Under the high potential difference, the atoms or molecules of gaseous substance are broken. The electrons in the outermost orbitals of these species are torn up. These electrons are repelled by the cathode, attracted by the anode and so travel from cathode towards the anode.
Since the nature of the cathode rays remain the same for every gas, it means that electron is essentially present in every particles of the matter. Low pressure is necessary to allow the cathode rays to reach safely to the anode.
Cathode rays and daily life
1. Cathode rays are used as advertisement neon sign.
2. Television pictures tube evolves cathode rays.
3. Monitor of computer is also a cathode rays tube.
DISCOVERY OF PROTONS (POSITIVE RAYS)
By using the discharge tube, E. Goldstein (1886) performed a series of experiments. He observed that there is fluorescence at the end of the tube opposite to the anode, when the cathode surface is made perforated. The rays coming from the anode pass through the holes of the cathode and strike the wall of the tube. These rays are called the anode rays, positive rays or canal rays.
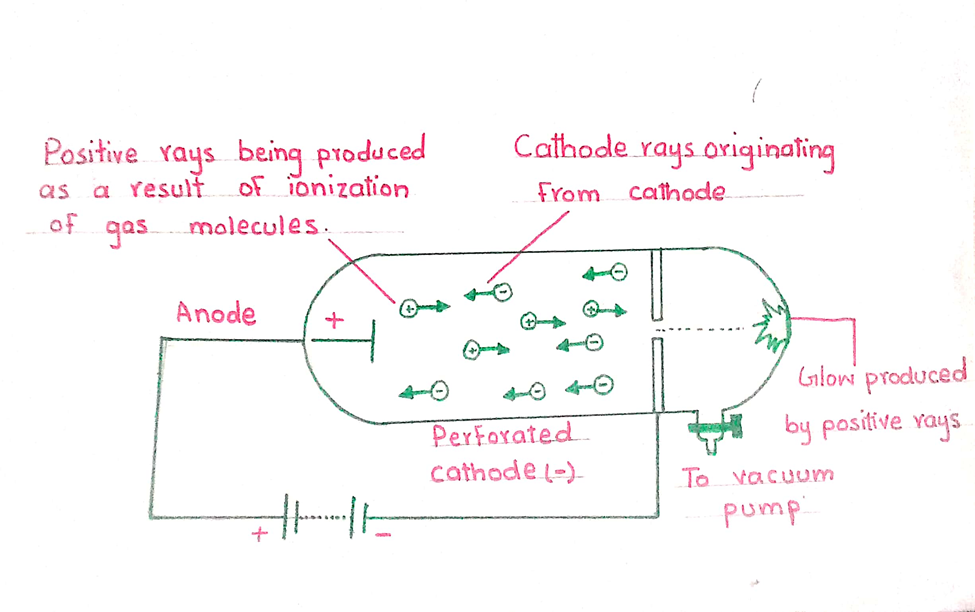
PROPERTIES OF ANODE RAYS
1. Way of travelling
These rays travel perpendicular to the surface of the anode.
2. Motion in a straight line
They travel in a straight line.
3. Effect of electric and magnetic field
They are deflected by the electric field towards the negative plate, showing that they are positively charged.
4. e/m value
Their e/m value is much smaller than the cathode rays. It means that their masses are much greater than electron.
5. Nature of gas
When different gasses are used in the discharge tube, then different anode rays with different e/m values are obtained.
6. Discovery of proton
When hydrogen gas is used in a discharge tube, then the positive rays of maximum e/m values are obtained. It means that the positive particles of anode rays cannot be less than the mass of protons.
Conclusion
1. Atom is divisible.
2. Proton is much heavier than that of electron.
3. The quantity of a positive charge on the proton is equal to the quantity of the negative charge on the electron.
How positive rays are produced?
Under the high voltage the molecules of a gas are dissociated into atoms at low pressure. When high speed electrons in the form of cathode rays strike these atoms, atoms are converted into cation.
M + 1e– —-> M+ + 2e–
These cations are positively charged icons, pass through the holes of the cathode and constitute the positive rays. The electrons obtained from the braking of atom or molecules move towards anode, and constitute the cathode rays.
IDEA OF PROTON IN ATOMS
When hydrogen gas is used in the discharge tube at low pressure then molecules of H2 are broken up into hydrogen atoms. Under high voltage these hydrogen atoms are deprived of their electrons. Protons are left behind as cation.
The positive charge of hydrogen ion (H+) is found to have1.6022 x10-19 coulombs of charge. This charge is just equal to charge of electron. e/m values of the H+ can be calculated. By substituting the value of ‘e’, we can get the mass of H+ which comes out to the 1.6726 x 10-27 kg. This positive ray particle or H+ is called proton.
DISCOVERY OF NEUTRON
Neutron was discovered by Chadwick in 1932. For this discovery he was awarded the Nobel prize in physics in 1935.
Experiment for discovery of neutron
Chadwick directed a stream of alpha- particle obtained from polonium. He found that the penetrating radiations were produced. The charge detector showed that these particles were neutral. He named these radiations as neutrons for this reason this figure this is a sort of nuclear reaction in which beryllium is converted to carbon.
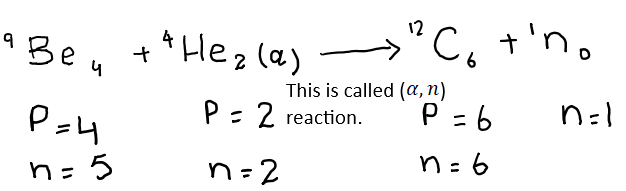
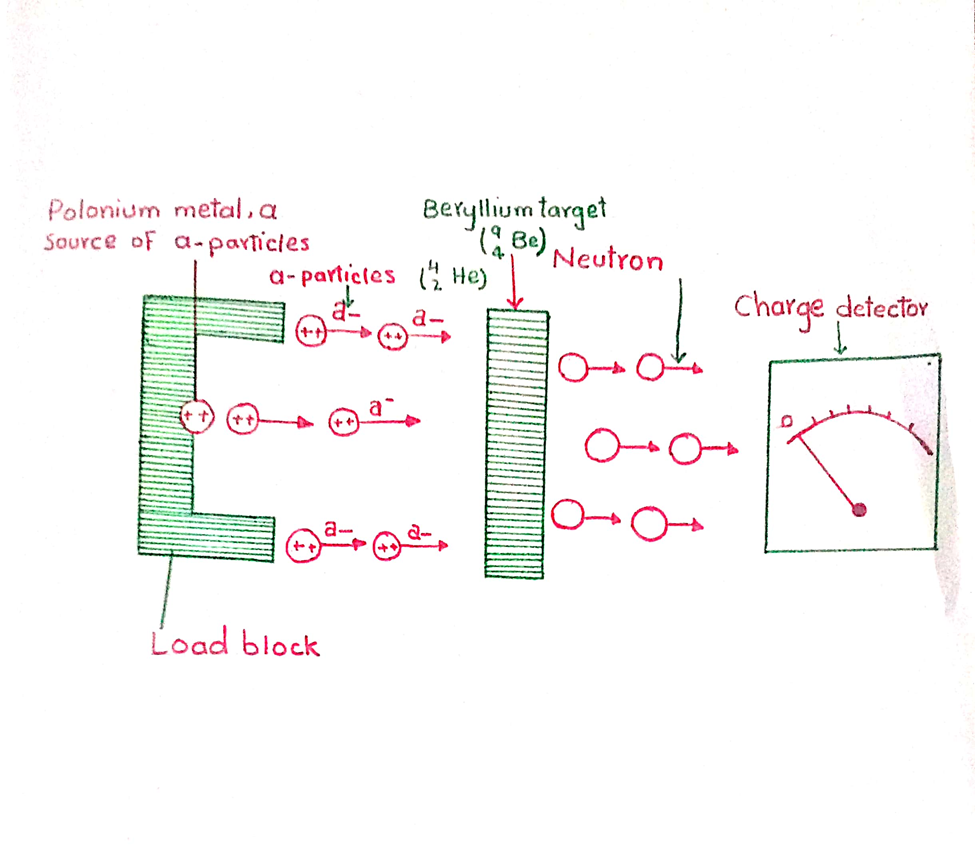
When beryllium atom and the alpha-particle interact with each other, they have collectively six protons and seven neutrons. In 12C6 , there are six proton and six neutrons. The seventh neutron is emitted in the form of radiation
Definition of neutron
It is a subatomic particle which bears a mass equal to that of proton (approximately) and has no charge on it. If the mass of proton is 1 a.m.u, then the mass of neutron is 1.00898 a.m.u. It is equal to 1.672 x 10-27 kg.
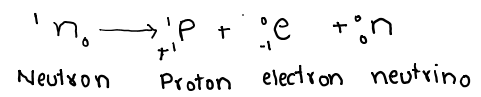
2. Neutrons are very penetrating.
3. Neutron can expel protons from paraffin, water and paper etc.
4. They do not produce any ionization.
5. When neutrons travel and have the energy of 1.2 Mev, then they are called fast neutrons. The neutron with energy less than 1.0ev are called slow neutrons. Slow neutrons are more effective that fast ones.
6. Neutron can act as projectiles. They can carry out the nuclear reaction. We can convert nitrogen to boron and alpha-particles are emitted.
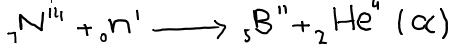
7. Slow moving neutrons hit the Cu metal and gamma-radiations of energy hv are emitted.
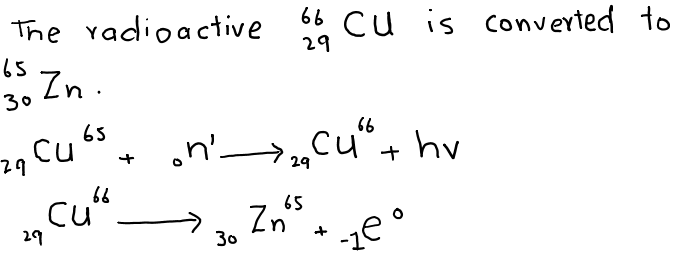
8. Neutrons have intense biological effects. They are being used in the treatment of cancer.