Learning objectives
In this article, the author has explained the following topics
- Distribution law
- Distribution coefficient
- Applications of distribution law
- limitations of distribution law
Distribution law or partition law
Table of Contents
At constant temperature, a solute distributes itself between two immiscible liquids in a constant ratio of concentration, independent of the amount of solute added.
Definition of distribution law
Two important techniques are totally based on the distribution law.
- Solvent extraction
- Partition chromatography
Distribution coefficient
At constant temperature, the ratio of the concentration of a substance in two immiscible liquids, present in equilibrium with each other, is called distribution co-efficient.
Definition of distribution coefficient
It is represented by K
If we talk about its mathematical expression then
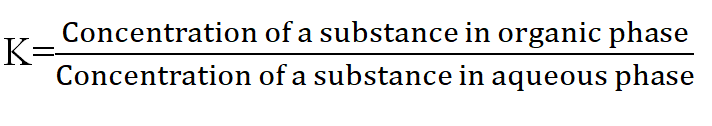
In above equation the organic phase may be a organic solvent such as CCl4 and aqueous phase is water.
Also read:
Experimental techniques in chemistry
How is distribution law modified by change in Molecular state?
Nernst’s Distribution Law
In 1891 Nernst studied the distribution of several solutes between different appropriate pairs of solvents. He gave a generalization which governs the distribution of a solute between two non-miscible solvents.
This is called Nernst’s distribution law.
If C1 denotes the concentration of the solute in solvent A and C2 the concentration in solvent B, Nernst’s distribution law can be expressed as:
C1/C2 = Kd
The constant Kd or simple K is called the distribution coefficient or partition coefficient or distribution ratio.
Examples of distribution law or partition law
Distribution of iodine between CCl4 and water containing KI
Consider the distribution of I2 between two immiscible liquids, CCl4 and water in the presence of Potassium iodide KI.
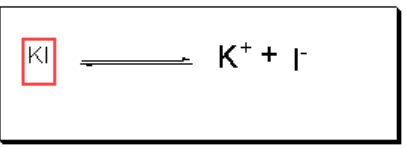
Iodine is insoluble in water while KI is added into water.
In water, KI ionizes into ions
Iodine combines with iodide ion to form tri-iodide ion in a reversible reaction.
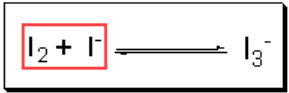
Thus iodine dissolves as I3– ion in water that is aqueous phase
Now if we add CCl4 to an aqueous solution that contains tri-iodide ion. As iodine is more soluble in CCl4 than in water therefore, it moves from aqueous layer to the CCl4 layer that is organic layer.
As a result, the brown color of tri-iodide ion in the aqueous layer fades while the purple color of free iodine appears in CCl4 layer.
This system of CCl4 and H2O is shaken to further increase the area of contact between the two layers. By doing so more and more iodine moves from the aqueous layer to the organic layer.
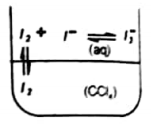
After repeating this process for some time, equilibrium is established between the two layers. At this point, the rate of movement of I2 from H2O to CCl4 becomes equal to the rate of movement of I2 from CCl4 to H2O.
Hence at equilibrium the ration of concentration of I2 in both layers will be constant at constant temperature. This constant is called distribution co-efficient and it is denoted by K.
Distribution co-efficient is represented as
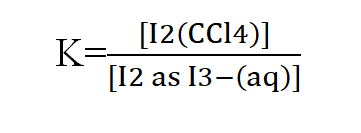
Solved example 2:
A solid X is added to a mixture of benzene and water. After shaking well and allowing to stand, 10 ml of the benzene later was found to contain 0.13 g of X and 100 ml of water layer contained 0.22 g of X. Calculate the value of distribution coefficient.
Solution
Concentration of X in Benzene (Cb)= 0.13/10 = 0.013 g ml-1
Concentration of X in water (Cw)= 0.22/100 = 0.0022 g ml-1
According to distribution law
Cb/Cw = 0.013/0.0022 = 5.9
Solved example 2
In the distribution of succinic acid between ether and water at 15 degree centigrade, 20 ml of the ethereal layer contains 0.092 g of the acid. Find out the weight of the acid present in 50 ml of the aqueous solution in the equilibrium with it if the distribution coefficient for succinic acid between water and ether is 5.2.
Solution
Let the weight of succinic acid in aqueous layer be X g.
Concentration in aqueous layer = X/50 g ml-1
Concentration in ethereal layer = 0.092/20 g ml-1
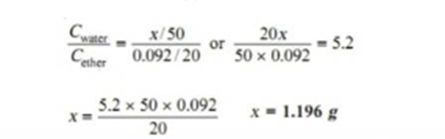
Solubilities and distribution law
When a solute is shaken with two non-miscible solvents at equilibrium both the solvents are saturated with the solute. Since the solubility also represents concentration we can write the distribution law as:
C1/C2=S1/S2=Kd
Where S1 and S2 are the solubilities of the solute in the two solvents.
Hence knowing the value of the distribution coefficient (Kd) and the solubility of the solute in one of the solvents the solubility of solute in the second solvent can be calculated.
Solved Problem
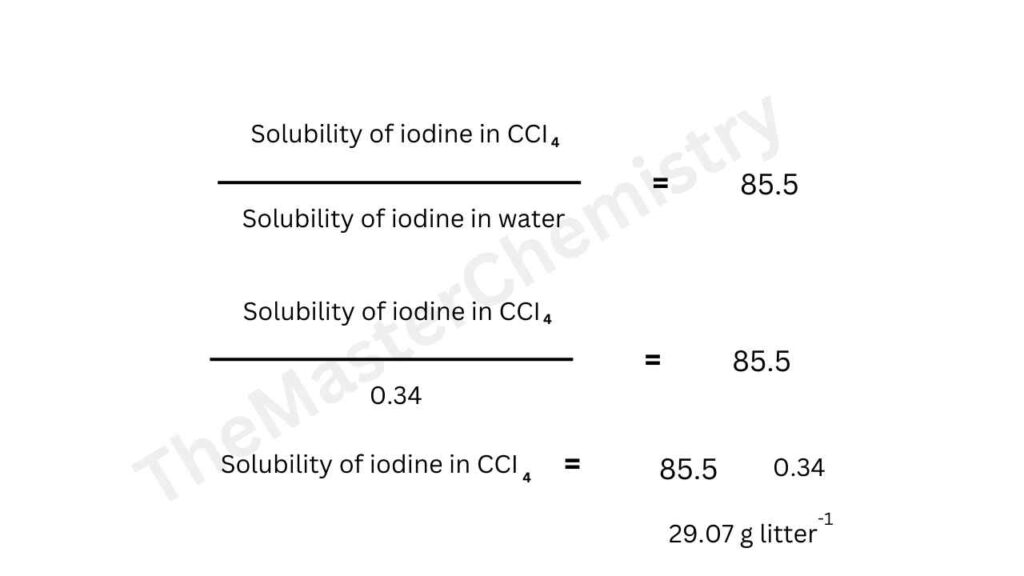
At 25 degree centigrade an aqueous solution of iodine containing 0.0516 g litre-1 is in equilibrium with a carbon tetrachloride solution containing 4.412 g litre-1. Find the solubility of iodine in carbon tetrachloride.
Solution
- To find the value of Kd
Concentration of Iodine in water= 0.0573 litre-1
Concentration of iodine in CCl4= 4.412 litre-1
The value of distribution coefficient is
Cccl4/CH2O= 4.412/0.0516= 85.5
- Calculation of Solubility S
Applying the distribution law
Applications of distribution law or partition law
This law is helpful in many ways in industrial chemistry and physics.
- It is much helpful in the separation and purification of substances from mixtures such as solvent extraction.
- It is useful to determine the extent of hydrolysis
- It also helps in the confirmation of the formula of the complexes such as CuSO4.4NH3
- It is used to find the equilibrium constant for the equilibrium
- It is used to determine the solubility of the different solutes in a solvent
- It is used for solvent removal purposes
- It is also helpful in the separation of high act liquid chromatography
- It is used to conserve the emulsions and creams
- It helps in the formation of the solubilized structure
- Used to determine the solubility of the different drugs in specific solvents
Limitations of distribution or partition law
Although it is very useful law, but also have some limitations:
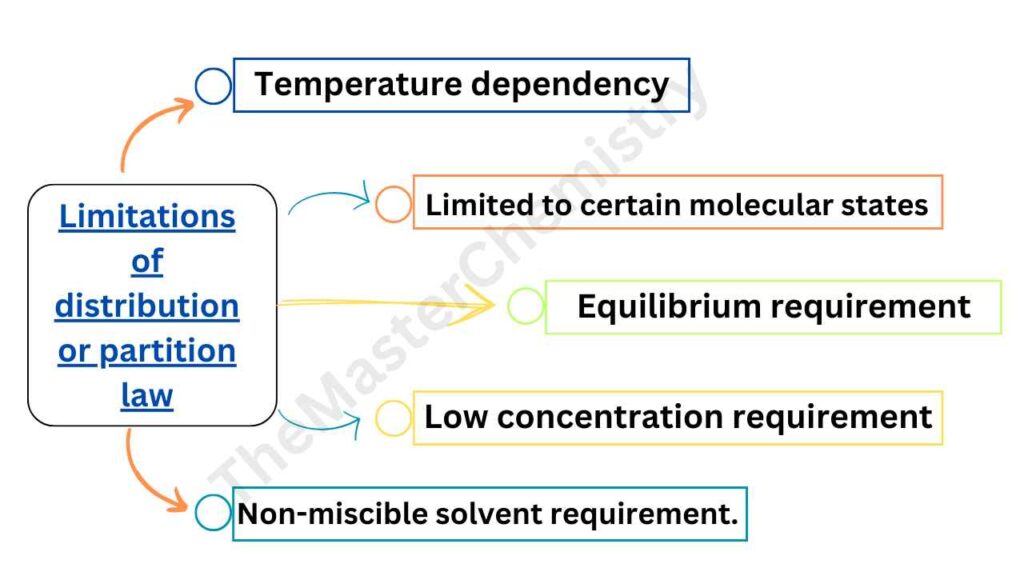
- It is not functional in variable temperature condition
- It does not describe the process of dissociation or association in different molecular state
- The concentrations of the solute can only be noted when the equilibrium is established.
- The concentration of the solute in the two solvents is low. This law does not hold when the concentrations are high.
- The two solvents are non-miscible or only slightly soluble in each other. The extent of mutual solubility of the solvents remains unaltered by the addition of solute to them.
Let others know about this