Learning Objectives
In this article, the author has explained about
- What is hydration energy
- Trends of hydration energy in periodic table
- Hydration energy and lattice energy
- Application of hydration energy
Hydration energy
Table of Contents
It is the amount of heat absorbed or evolved when one mole of gaseous ions dissolve in water to give an infinitely dilute solution.
Definition of Hydration energy
It is also known as hydration enthalpy. This is a special case in the condition when water is used as a solvent.
Hydration energy vs Lattice enthalpy
When we dissolve salt into water, the ions present at the edge of the lattice (Outermost ions) start moving away from the lattice. After separation from the lattice, these ions are covered by the solvent (water) molecules.
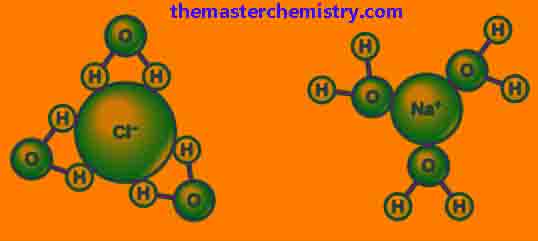
At this point if the hydration energy is equal to or greater than the lattice energy, then the solute (salt) is said to be water-soluble. For such salts energy is released in the form of heat.
Dissolvation of CaCl2 is the perfect example of this process. Because when CaCl2 is dissolved into water, the water becomes hot because it releases the energy which means that hydration energy completely overcomes the lattice energy.
Examples:
When one mole of gaseous hydrogen ions is dissolved in water to give an infinitely dilute solution then 1075 KJ of energy is evolved. Therefore, it is called the hydration energy of H+ ion.
Factors affecting the hydration energy
Size of the ion
When an positively charged ion, has smaller size then the charge density is also smaller. Similarly the forces of attractions between the positive ion and the water molecules are also smaller. Therefore, the hydration energy is becomes smaller.
Amount of the charge
With greater amount of positive charge on the ion, the charge density is also increases. Therefore, the hydration energy is greater in this case.
Nature of ion
Negative ions also show the hydration energies. Sizes of the negative ions are mostly greater than the positive ions. For this reason the hybridization energy of negative ions are smaller.
The table below shows the values of the hydration energies for some of the positive and negative ions which mostly make the ionic bonds.
The ionic compounds are dissociated in water and they are dispersed in the solution.
Trends of hydration energy in the periodic table
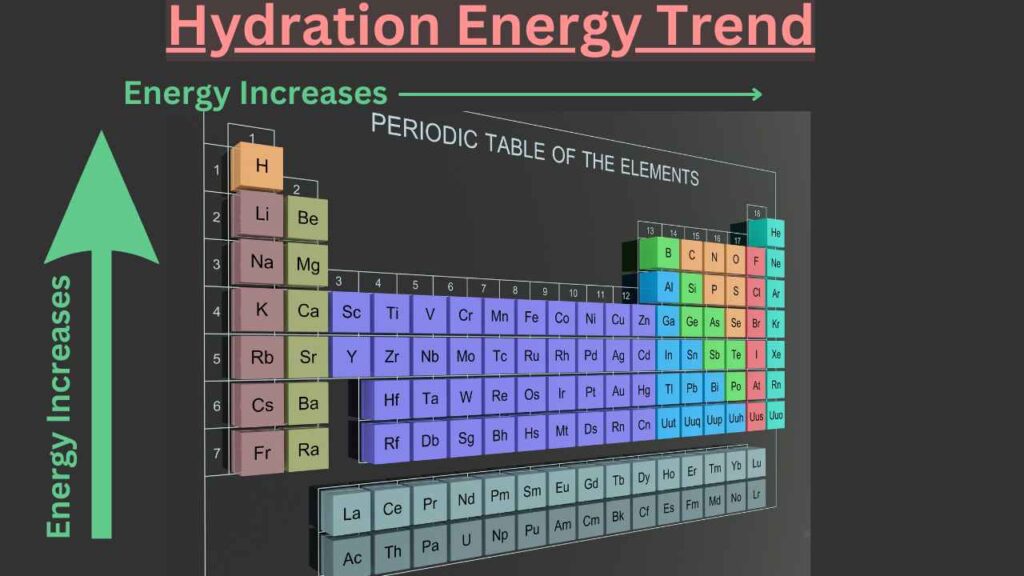
Trends of hydration energy in the group
As we know that the amount of positive or negative charges remain the same down the group therefore, the charge densities decrease. For this reason, hydration energies decrease down the group.
Trends of hydration energy in the period
The values of the hydration energies increase from left to right in a period for isoelectronic species.
Species having an equal number of electrons are known as isoelectronic species
For example, Na+, Mg+2, and Al+3 have ten electrons each. These are isoelectronic species. The hydration energies increase with the increasing positive charge.
In the case of elements of 3rd period the elements such as K+ and Ca+2 the hydration energies increase. They are both isoelectronic. Ca+2 has greater charge density than K+, so Ca+2 has five times greater hydration energy than K+.
Hydration energies of Different ions
| Ion | ΔHhyd |
| H+ | -1130 |
| Li+ | -520 |
| Na+ | -406 |
| K+ | -322 |
| Rb+ | -297 |
| Cs+ | -276 |
| Ion | ΔHhyd |
| Al3+ | -4465 |
| Be2+ | -2494 |
| Mg2+ | -1921 |
| Ca2+ | -1577 |
| Sr2+ | -1443 |
| Ba2+ | -1305 |
Negative hydration energies
| Ion | ΔHhyd |
| F- | -1130 |
| Cl- | -520 |
| Br- | -406 |
| I- | -322 |
| ClO4 | -297 |
Application of hydration energy

The most important application of hydration energy or enthalpy setting of cement during construction. It is known as the reaction of cement with water which is exothermic in nature.
During this process, a large amount of heat is released that can cause cracks in the building because during the setting process heat released makes the outer surface of bricks or blocks much faster than the inner surface.
Therefore, the engineers prefer using ice instead of water while preparing cement mixture. Moreover, fly ash, slag can also be add to avoid this issue.
Let others know about this