What is Friedel-Craft acylation?
Table of Contents
Addition of acyl group into Benzene ring is known as Friedel-Crafts acylation.
Usually, an acid chloride (R-(C=O)-Cl) and a Lewis acid catalyst like AlCl3 are used to do this. A ketone is created from the aromatic ring in a Friedel-Crafts acylation process. Below is an illustration of how benzene and an acyl chloride react in these circumstances.
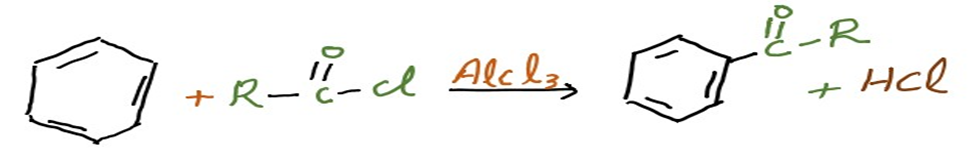
In Friedel-Crafts acylation, an acid anhydride can be used instead of an acyl halide. By forming a complex with the Lewis acid, the acyl halogen creates a highly electrophilic acylium ion with the general formula RCO+ that is stabilized by resonance.
Friedel Craft Acylation Mechanism
Step 1: Formation of acylium ion
The acyl halide and the Lewis acid catalyst (AlCl3) engage in a reaction. The acyl halide loses a halide ion as a result of the formation of a complex, creating an acylium ion that is stabilized by resonance.
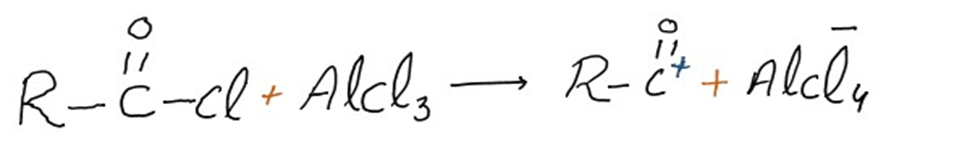
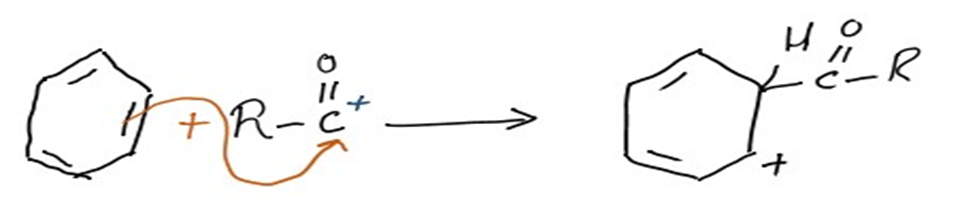
Step 2: Electrophilic attack forms a sigma complex
The aromatic ring is then attacked electrophilically by the acylium ion (RCO+). As a complex develops, the ring’s aromaticity is momentarily lost.
Step 3: Loss of proton regenerates the aromatic ring
The intermediate complicated is now deprotonated, restoring the aromaticity to the ring. This proton attaches itself to a chloride ion (from the complexed Lewis acid), forming HCl. The AlCl3 catalyst is now regenerated.
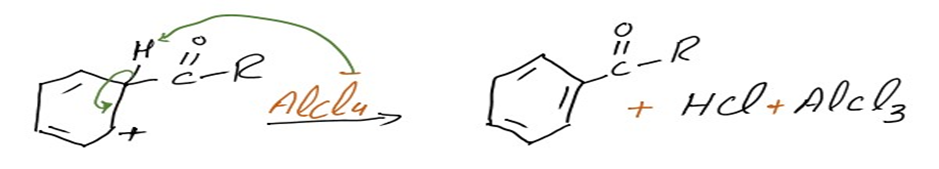
Thus, the required acyl benzene product is obtained via the Friedel-Crafts acylation reaction.
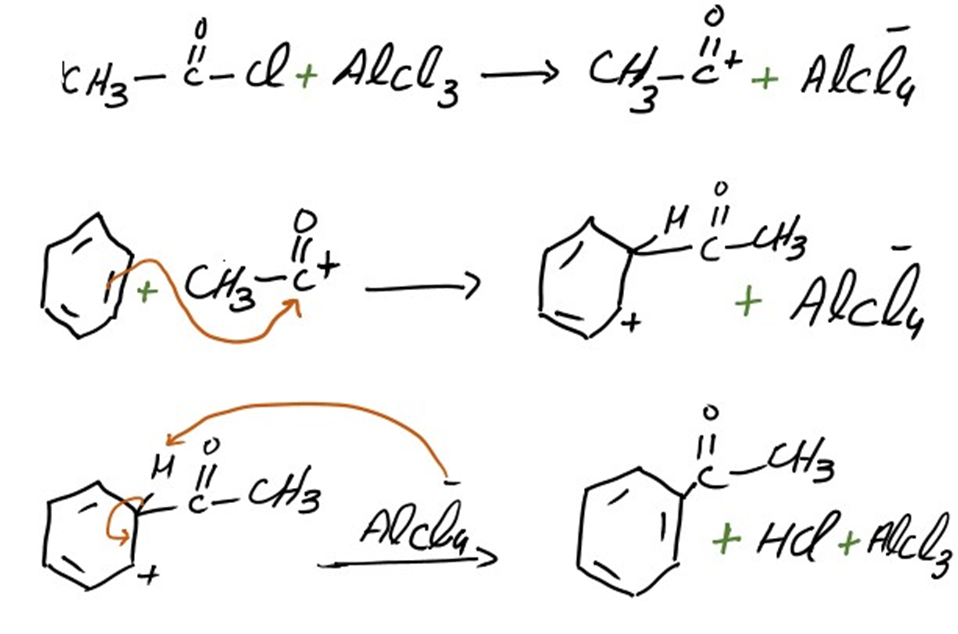
Addition of acetyl group
The mechanism of addition of acetyl group into benzene is same as we discuss above. Acetophenone is formed as a result of this addition.
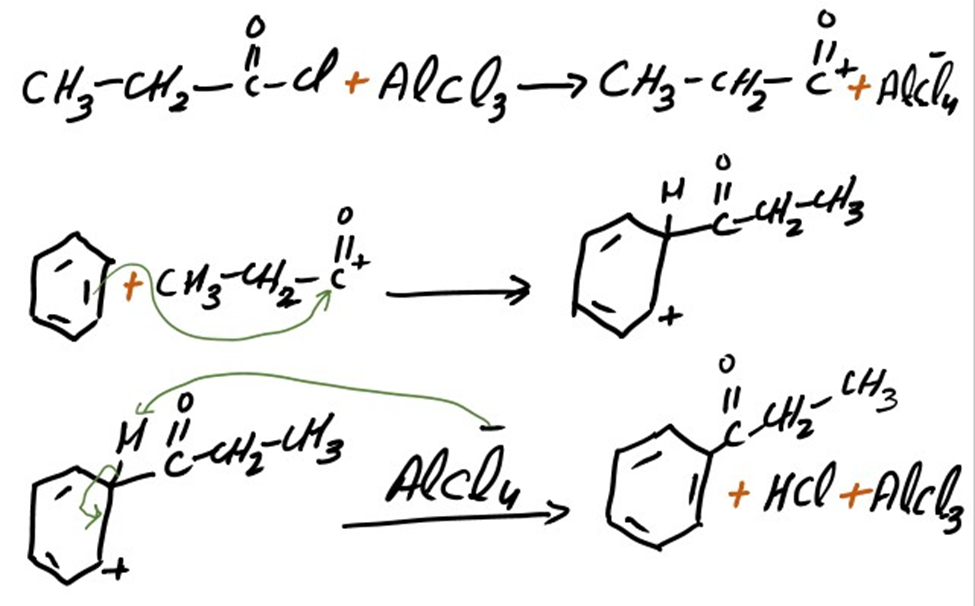
Addition of propanoyl group
Propiophenone is formed as a result of addition of propanoyl group. The mechanism of addition of propanol group into benzene is same as we discuss above.
Limitations of Friedel Craft Acylation
Despite overcoming some barriers of the related alkylation response (such as carbocation rearrangement and polyalkylation), the Friedel-Crafts acylation response has a few shortcomings.
The acylation response solely yields ketones. This is due to the fact formyl chloride (H(C=O)Cl) decomposes into CO and HCl when uncovered to these conditions.
The fragrant compound can’t take part in this response if it is much less reactive than a mono-halobenzene.
Aryl amines can’t be used in this response due to the fact they structure fairly unreactive complexes with the Lewis acid catalyst.
The acylation can take region on the nitrogen or oxygen atoms when amine or alcohols are used. Thus, the response details, mechanisms, and boundaries of each Friedel-Crafts reactions are temporarily discussed.
FAQS
What are the advantages of Friedel Crafts acylation?
Friedel Crafts Acylation have a number of advantages over Friedel Craft Alkylation. These blessings encompass a higher manipulate over the response merchandise and additionally the acylium cation is stabilized with the aid of resonance so no possibilities of rearrangement. Using Clemmensen reduction, the ketones made can be decreased to alkyl groups.