Written By Adeel Abbas
Gas Chromatography (GC) is a powerful analytical technique used to separate and identify individual components in a mixture of gases or volatile liquids.
The technique of chromatography is widely used in a variety of fields, including chemistry, environmental science, and forensic science.
Gas chromatography is a method of separation in which the components of a mixture are distributed between a stationary phase and a mobile phase. The stationary phase is a solid or a liquid, while the mobile phase is a gas. The components of the mixture are separated based on their chemical and physical properties, such as boiling point, vapor pressure, and polarity.
Principal of gas chromatography
Table of Contents
The basic principle of gas chromatography is that different compounds in a mixture will have different interactions with the stationary phase, and will therefore travel at different rates through the column.
The compound that interacts least with the stationary phase will travel through the column the fastest, while the compound that interacts most with the stationary phase will travel through the column the slowest. This results in the separation of the compounds in the mixture.
Example
For example, let’s say we have a mixture of three compounds: A, B, and C. Compound A has a higher boiling point than compounds B and C, so it will interact more with the stationary phase and travel through the column more slowly. Compound B has a lower boiling point than compound A but higher than compound C, so it will travel through the column at a faster rate than compound A, but slower than compound C. Compound C, having the lowest boiling point, will travel through the column the fastest.
Mobile phase and stationary phase in gas chromatography
| Mobile Phase | Stationary Phase |
| Helium | Polydimethylsiloxane (PDMS) |
| Nitrogen | Polyethylene glycol (PEG) |
| Hydrogen | Porous polymer beads (e.g. Tenax) |
| Argon | Carbon-based materials (e.g. Graphitized Carbon) |
| Carbon dioxide | Cyclodextrins |
| Methane | Metal-organic frameworks (MOFs) |
| Oxygen | Silica gel |
| Air | Alumina |
| Propane | Polystyrene divinylbenzene (PS-DVB) |
| Butane | Cyanopropylphenyl polysiloxane |
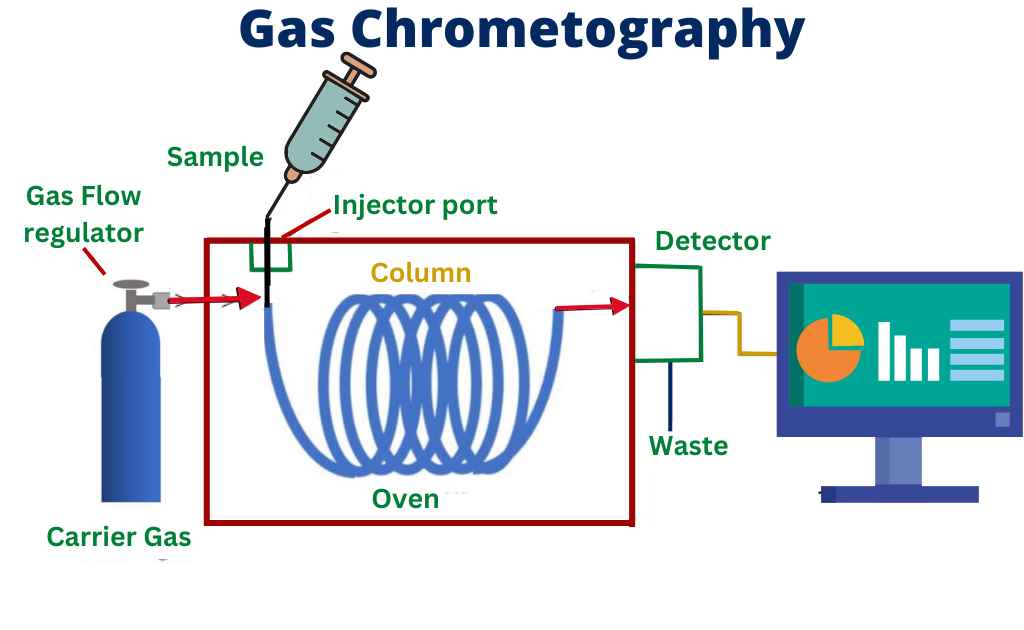
Instrumentation of gas chromatography
In gas chromatography, the key components of the instrumentation include the injector, the separation column, the detector, and the data system.
Injector: The injector is used to introduce the sample into the gas chromatograph. The most common types of injectors are the split injector and the on-column injector. The split injector is the most widely used and allows for the introduction of a large sample volume into the column. The on-column injector is used for trace analysis and allows for the introduction of a smaller sample volume into the column.
Separation Column: The separation column is where the actual separation of the compounds in the sample occurs. The column is typically made of a long, thin tube filled with a stationary phase. The stationary phase can be a liquid or a solid, and is chosen based on the properties of the compounds being analyzed. The most common types of stationary phases are polar and non-polar, and they are used to separate the compounds in the sample based on their polarity.
Detector: The detector is used to detect the separated compounds as they exit the column. The most common types of detectors used in gas chromatography are flame ionization detectors (FID), thermal conductivity detectors (TCD) and mass spectrometry (MS). The FID is sensitive to all types of compounds and is the most widely used detector. The TCD is sensitive to non-volatile compounds and is used for trace analysis. Mass spectrometry is a powerful detector that allows for the identification and quantification of the compounds in the sample.
Data System: The data system is used to collect, process, and display the data generated by the gas chromatograph. The data system typically includes a computer, a data acquisition system, and software for data processing and analysis.
In summary, the instrumentation of gas chromatography includes the injector, which is used to introduce the sample into the chromatograph, the separation column, where the actual separation of the compounds occurs, the detector, which is used to detect the separated compounds and the data system, which is used to collect, process and display the data generated by the gas chromatograph.
Application of gas chromatography
Gas chromatography (GC) is a powerful analytical technique that is widely used in a variety of fields, including chemistry, environmental science, and forensic science. Some of the most common applications of GC include:
Analysis of volatile organic compounds (VOCs) in air and water: GC is commonly used to measure the concentrations of VOCs in air and water, such as benzene, toluene, and xylene. This information can be used to assess air and water quality and identify sources of pollution.
Analysis of food and flavorings: GC is used to identify and quantify the various compounds present in food and flavorings. This information is used to ensure the quality of food products, as well as to develop new flavors and fragrances.
Analysis of pesticides and other toxins in food and water: GC is used to detect and quantify the presence of pesticides and other toxins in food and water. This information is used to protect public health and ensure the safety of food and water supplies.
Analysis of petrochemical products: GC is used to analyze a wide range of petrochemical products, including gasoline, diesel fuel, and lubricating oils. This information is used to ensure the quality of these products and to identify potential problems such as contamination.
Analysis of drugs and their metabolites in biological fluids: GC is used to detect and quantify the presence of drugs and their metabolites in biological fluids, such as blood and urine. This information is used in forensic science and in the monitoring of drug therapy.
Analysis of environmental pollutants: GC is used to analyze pollutants in the environment such as polychlorinated biphenyls (PCBs) and polyaromatic hydrocarbons (PAHs) in soil and water.
Analysis of essential oils and fragrances: GC is used to identify and quantify the individual compounds present in essential oils and fragrances. This information is used in the production and quality control of these products.
Analysis of gases: GC is used to analyze a wide range of gases such as methane, propane, and carbon dioxide. This information is used in industries such as natural gas production, petrochemical, and fuel storage.
In conclusion, Gas chromatography (GC) is a powerful analytical technique that is widely used in a variety of fields such as chemistry, environmental science, and forensic science. Its ability to separate and identify individual components in a mixture of gases or volatile liquids makes it a valuable tool for analyzing and monitoring a wide range of substances, from volatile organic compounds, food and flavorings, pesticides, petrochemical products, drugs, environmental pollutants, essential oils, fragrances, and gases.