LEARNING OBJECTIVES
In this article, author has explained the introduction, functions and examples of amphipathic molecules.
Definition
Table of Contents
The molecules which have both polar and non-polar ends are called amphipathic molecules. This characteristic gives them both hydrophobic and hydrophilic (water-loving) properties. Amphipathic molecules are lipophilic (fat-loving)
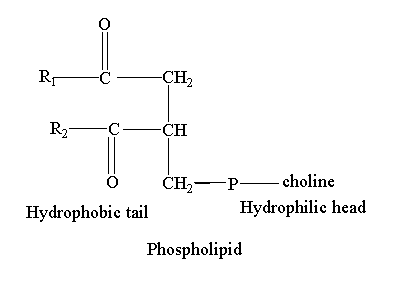
For example, phospholipids have a polar phosphate head and a non-polar hydrocarbon tail. Sphingolipids, bile salts, cholesterol, and fatty acid are also amphipathic molecules.
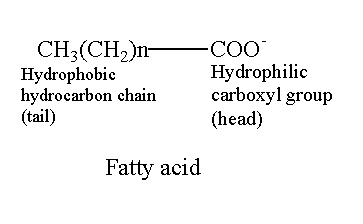
Similarly, the fatty acid is also amphipathic. Fatty acid contains a carboxyl group (COO–) and hydrocarbon chain. The Carboxyl group is polar with an affinity to attract water while the hydrocarbon chain is hydrophobic or non-polar.
Functions of amphipathic molecules
- The most important function of amphipathic molecules is the formation of the plasma membrane. The phospholipids head makes the external side of the membrane while the hydrocarbon chain makes the internal side of the membrane. So, the inside of the membrane is hydrophobic. This arrangement is critical for the movement of substances from inside to outside or outside to inside.
- Small non-polar molecules can pass easily through the membrane along their concentration gradient whereas polar molecules cannot pass through it. The hydrophobic lipid bilayer forms a barrier between the outside and inside of the membrane.
- Polar molecules such as water and certain proteins are needed to move inside the membrane. This transportation is carried by membrane proteins. Membrane proteins are amphipathic.
- Cholesterol is also amphipathic. It is an important component of the membrane of animal cells. It maintains the fluidity of the membrane and provides structural integrity to animal cells. Cholesterol maintains the shape of animal cells and allows animals to move. In addition to this, cholesterol is also responsible for selective permeability of the membrane, cell signaling, intracellular transport, and nerve conduction.
- A micelle is an aggregation of surfactant molecules where a hydrophilic head region is oriented outwards whereas a hydrophobic tail region is centered inside. It forms a spherical shape. Bile acids form micelle due to their amphiphilic nature. Bile acids which contain micelle help in the digestion of lipids.
- When amphipathic lipids in an aqueous medium are subjected to sonification, liposomes are produced. Liposomes in combination with specific antigens are used as the carrier of drugs to target tissues.
- When non-polar lipids are mixed with water emulsions are produced. These are large and stabilized by emulsifying agents. The common emulsifying agents are amphipathic lipids such as bile salts and phospholipids.
Examples of amphipathic molecules
1. Phospholipids
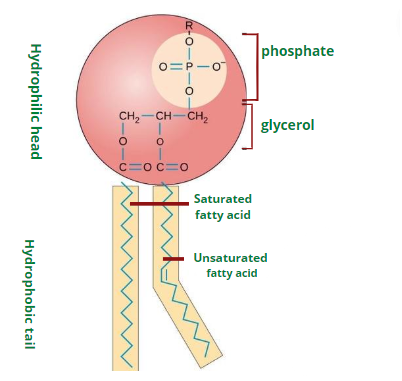
Phospholipids consist of glycerol attached to two fatty acids and a phosphate group. The glycerol with negatively charged phosphate group is a hydrophilic part of phospholipid. The two long fatty acid chains are the lipophilic hydrophobic region of phospholipids.
The amphipathic nature of phospholipid makes it the component of biological membranes. The plasma membrane is made up of two layers of phospholipids. Phospholipids interact with various molecules depending upon polarity. The hydrophilic head can interact with the polar molecules while the hydrophobic tail avoids polar interactions. This characteristic makes the plasma membrane selective and permeable.
2. Cholesterol
Cholesterol is also an amphipathic molecule. The OH group present in it is hydrophilic while the steroid and hydrocarbon chains are hydrophobic. Cholesterol is present in the plasma membrane of animals. The hydrophilic portion interacts with the water and with the hydrophilic heads of phospholipids. The hydrophobic tails are embedded in the membrane.
3. Glycolipids
Glycolipids consist of hydrophilic sugar groups and hydrophobic lipid chains. Therefore, glycolipids are amphipathic. Glycolipids are also present in the membrane. The carbohydrate region is arranged towards the outside while the lipid region is embedded inside the membrane. The sugar interacts with the polar molecules. The carbohydrate portion extended outwards also allow carbohydrate-carbohydrate interaction.
4. Bile acids
Bile acids have a steroid structure consisting of four rings, carboxyl, and hydroxyl groups. Bile acids form micelle by aggregating around the lipids. They act as a surfactant. They emulsify lipids; prevent small lipids to aggregate into large lipids.
5. Saponins
Saponins are amphipathic glycosides found in plants. They consist of hydrophilic glycoside and a hydrophobic steroid. Saponins are bitter that making plants less herbivory.
6. Amphipathic proteins
Amphipathic proteins also consist of polar and non-polar regions of amino acids. The hydrophilic portion consists of polar amino acids like asparagines and serine while the hydrophobic region consists of non-polar amino acids like glycine and proline. Membrane proteins are amphipathic proteins.
7. Soaps
Amphipathic molecules allow the soaps and detergents to carry out washing and cleaning. Soaps are made by treating with fatty acids like animal fats and vegetable oils and a chemical called lye. Lye is an ionic compound like salt. Lye creates a polar head on the fatty acid. Thus, soap can bind to both greases and carry away with the water.