LEARNING OBJECTIVES
In this article, author has explained the introduction, structure, physical and chemical properties, classification and functions of lipids.
Definition
Table of Contents
Lipids are heterogeneous compounds that are insoluble in water and soluble in organic solvents. Lipids are a major storage form of energy in the body. They naturally occur in plants, animals, and microorganisms. They are mainly composed of hydrocarbons.
Triglycerides, phospholipids, cholesterol, and fatty acids are few examples of lipids.
Structure of lipids
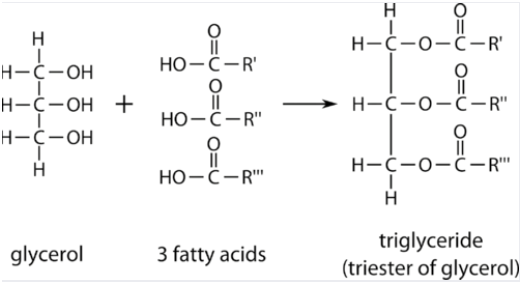
Lipids contain hydrogen, oxygen, and carbon. Unlike carbohydrates, lipids are not polymers. They are made of two molecules; glycerol and fatty acids.
Glycerol contains three carbon atoms with an OH group attached to it.
Fatty acid contains an acid group at one end of the molecule and hydrocarbon chain which is represented by R.
Triglycerides contain one glycerol attached to three fatty acids. The bond between them is a covalent and ester bond.
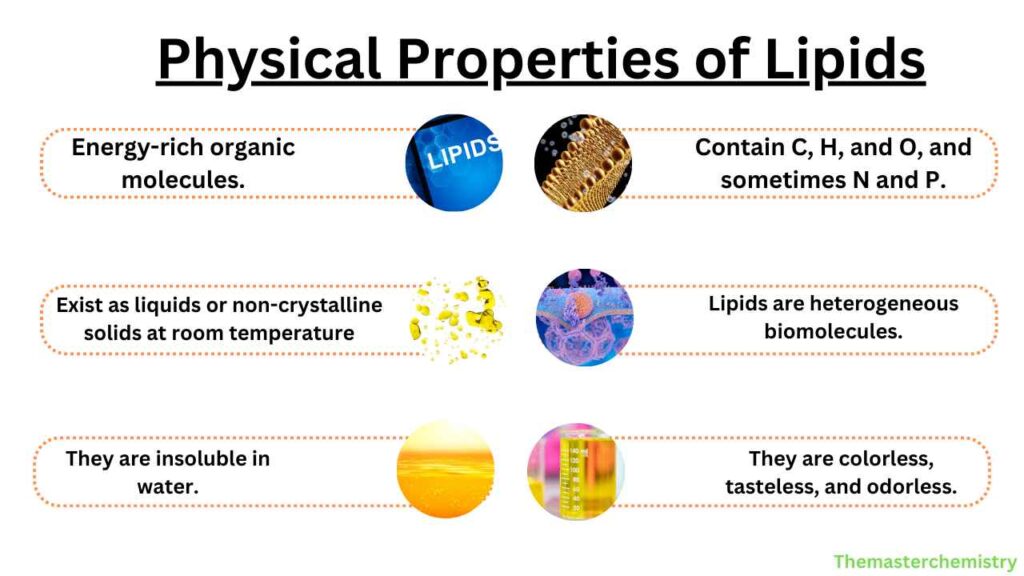
Physical Properties of lipids
- Lipids are energy-rich organic molecules.
- They contain carbon, hydrogen, and oxygen sometimes also contain nitrogen and phosphorus.
- They exist as liquids or non-crystalline solids at room temperature.
- They are heterogeneous biomolecules.
- They are colorless, tasteless, and odorless.
- They are insoluble in water.
- They are organic compounds soluble in organic solvents like acetone, benzene, and chloroform.
Chemical properties of lipids
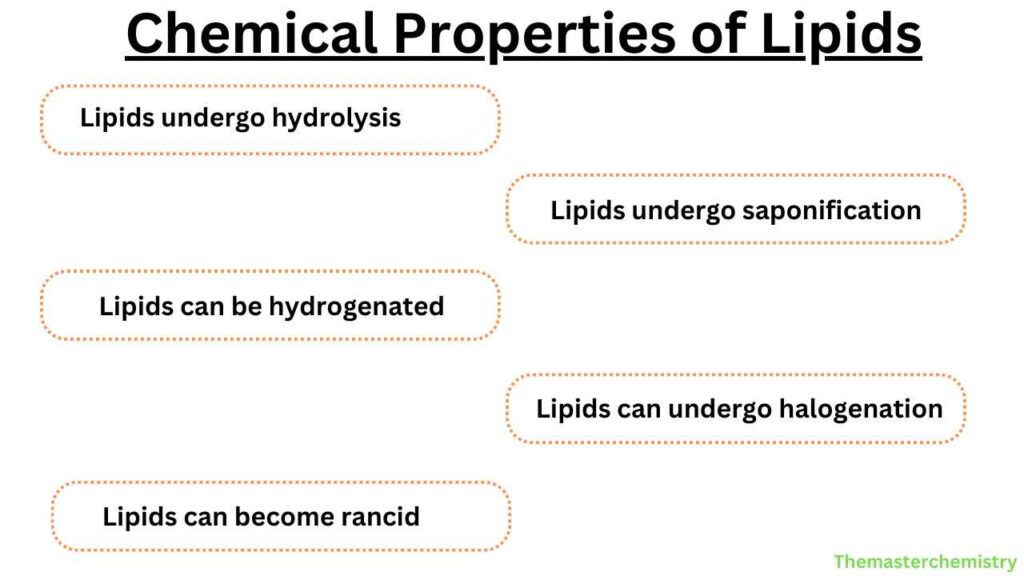
1. Hydrolysis of lipids
Triglycerols react with water and form carboxylic acid and alcohol. This process is known as the hydrolysis of lipids.
2. Saponification
The alkaline hydrolysis of lipids is known as saponification. This process utilizes alkali or enzymes such as lipases. This process is known as saponification because one of its products is soap.
3. Hydrogenation
When hydrogen is added to the unsaturated fatty acid to produce a saturated fatty acid, this process is termed hydrogenation.
4. Halogenation
Unsaturated fatty acids react with halogens. The halogen is added to a double bond. This reaction is known as halogenation. It results in the decolorization of the solution.
5. Rancidity
The term rancidity refers to disagreeable odor. Hydrolysis and oxidation of fatty acids cause rancidity. Oxidative rancidity occurs in the triacylglycerols that contain unsaturated fatty acids.
Classification of lipids
Lipids are divided into many classes which are further subdivided.
1. Simple lipids
Esters of fatty acids and alcohol are called simple lipids. Simple lipids are further classified into two classes.
Fats and oil are also known as triacylglycerols because they are esters of fatty acids and glycerol. Fats are saturated; they are solid at room temperature while oils are unsaturated; they are liquid at room temperature.
Waxes are esters of fatty acids and alcohol. Glycerol is not present in them. The alcohol present may be aliphatic and alicyclic. Waxes are used in the production of many products like candles, lubricants, cosmetics, and polishes. Cetyl alcohols are the most common alcohols found in waxes.
2. Complex/compound lipids
Complex/compound lipids are esters of fatty acids and alcohols with the additional group present. The additional group can be phosphate, nitrogenous base, carbohydrate, and protein.
Complex lipids are further divided on the base of the additional group present.
Phospholipids
Phospholipids are those lipids that contain phosphoric acid or nitrogenous base as an additional group. Phospholipids are further classified into two classes.
- Glycerophospholipids are those phospholipids that contain glycerol alcohol.
For example, lecithin and cephalin
- Sphingophospholipids are those phospholipids that contain sphingosine alcohol. For example, sphingomyelin is a sphingophospholipid.
Glycolipids
The lipids which contain carbohydrates covalently attached to lipids are called glycolipids. Glycolipids are also known as glycosphingolipids because they contain sphingosine alcohol. Glycolipids do not have phosphate and glycerol.
e.g; cerobrosides, gangliosides.
Lipoproteins
Those lipids which contain proteins attached to them are called lipoproteins.
LDL and HDL are examples of lipoproteins.
There are many other complex lipids; sulfolipids, amino lipids, and lipopolysaccharides.
3. Derived lipids
Lipids that are derived from simple and complex lipids are called derived lipids. They are derived from simple and complex lipids by hydrolysis.
Steroids, sterols, cholesterol, bile acids, etc are examples of bile acids.
4. Miscellaneous lipids
Miscellaneous lipids molecules that share some characteristics with lipids but do not fit into the main lipid categories like fats, oils, and phospholipids. These lipids are found in cell membranes, signaling pathways, and energy storage.
For example, squalene, terpenes, hydrocarbons, and carotenoids are miscellaneous lipids
5. Neutral lipids
Neutral lipids are a type of lipid that is mainly used for energy storage in living organisms. They are uncharged lipids (has no charge), that’s why are referred to as neutral lipids. Unlike other lipids, such as phospholipids that form cell membranes, neutral lipids are not involved in structural roles.
Monoacylglycerols, diacylglycerols, triacylglycerols, cholesterol are neutral lipids.
Functions of Lipids
Lipids are involved in following functions:
- Energy Storage
- Temperature Regulation
- Hormone Regulation
- Reproductive System Support
- Brain Function
- Organ Protection
- Insulation and Protection
- Vitamin Absorption
- Cell Membrane Structure
- Chemical Messengers
Sources of Lipids
- Oils (Canola, Corn, Olive, Peanut, Safflower, Soy, Sunflower oil) [source]
- Fats (Animal meat, Dairy products, Cocoa butter)
- Omega-3 Fatty Acids (Fatty fish (Salmon, Sardines, Herring), Flaxseeds, Walnuts, Soybean oil, Chia seeds)
- Omega-6 Fatty Acids (Various foods, especially abundant in walnuts)
- Avocados [source]
- Nuts and Seeds (Almonds, Pistachios, Sesame seeds)
- Coconut and Coconut Oil
- Egg Yolk
- Butter and Ghee
- Dark Chocolate
- Fatty Meat (Beef, Lamb, Pork, Poultry)
- Cheese