LEARNING OBJECTIVES
In this article, author has explained the structure of nucleotides, different types of nitrogenous bases and functions of nucleotides.
Definition
Table of Contents
Nucleotides are organic compounds that make up nucleic acids. They are building blocks of DNA. Nucleotides are essential parts of DNA, RNA, and cell function and perform many functions based on their structure. Nucleic acids are polymers of nucleotides.
Structure of Nucleotides
Each nucleotide is made of three components:
- Nitrogenous base
- Pentose sugar
- Phosphate
These three components make the structure of nucleotides. When many nucleic acids come together they made nucleic acids.
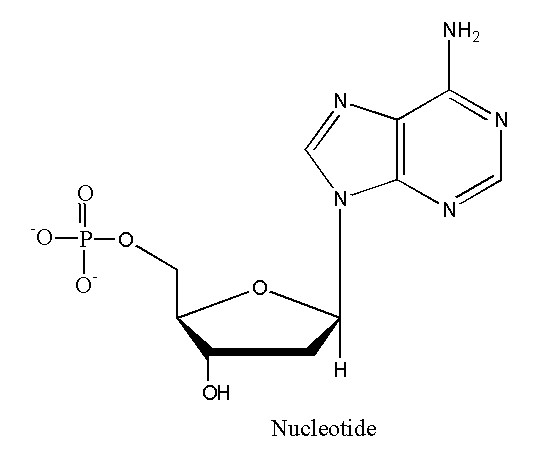
Nucleotides are linked together by phosphodiester bonds. The phosphate group acts as a bridge connecting two nucleotides together.
Nitrogen base
Nitrogen bases are of two types:
- Purines
- Pyrimidines
1. Purines
Purines are heterocyclic organic compounds containing two-hydrogen nitrogen ring bases and four nitrogens. Adenine and guanine are two nitrogenous bases that are purines. The melting point of purines is 2140C.
There are two types of purine bases:
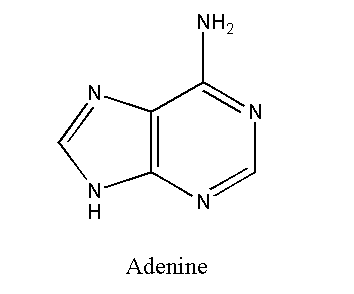
Adenine
Adenine (C5H5N5) is a purine. Purines have a double-ringed structure. Adenine bonds with thymine in DNA. In RNA, adenine bonds with uracil. Adenine links by two hydrogen bonds. These hydrogen bonds stabilize the structure of nucleic acid.
The adenine-containing nucleotide is called adenosine. Adenosine triphosphate (ATP) has also adenine as a nitrogenous base. ATP contains three phosphate groups so it can store large amounts of energy.
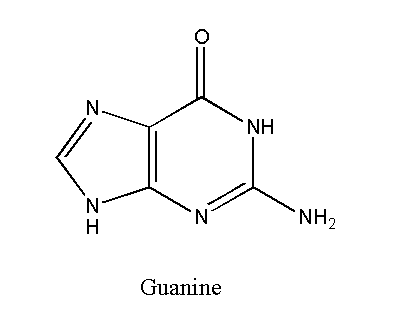
Guanine
Guanine is also a purine nucleotide with the chemical formula C5H5N5O. It has a double-ringed structure. Guanine binds with the cytosine in both DNA and RNA. Guanine binds with cytosine through three hydrogen bonds to form a nucleic acid. Therefore, a guanine-cytosine bond is stronger than an adenine-thymine bond.
The guanine-containing nucleotide is called guanosine. It has conjugated double bonds.
2. Pyrimidines
Pyrimidines are heterocyclic aromatic compounds that are composed of carbon and hydrogen. It also contains four nitrogens. There are three pyrimidines; cytosine, thymine, and uracil. It consists of one hydrogen-carbon ring. The melting point of pyrimidines is 20-220C.
There are three types of pyrimidines bases:
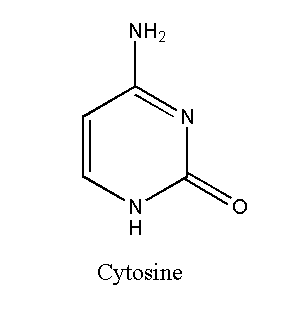
Cytosine
Cytosine is a pyrimidine nucleotide with the chemical formula C4H5N3O. It contains one ring structure. Cytosine bonds with guanine in DNA and RNA. Cytosine bonds with guanine through three hydrogen bonds. Cytosine-Guanine is a strong pair. A cytosine-containing nucleotide is called cytosine. Cytosine can also work as a co-enzyme that converts ADP (adenosine diphosphate) to ATP (adenosine triphosphate).
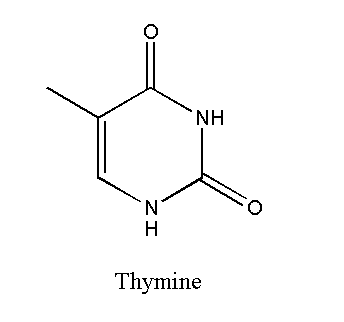
Thymine
Thymine is a pyrimidine with the chemical formula C5H6N2O2. Thymine bonds with adenine through two hydrogen bonds. This makes the thymine-adenine bond weaker. Thymine contains one ring with conjugated bonds. A thymine-containing nucleotide is called thymidine. Thymine is only present in DNA. It is absent in RNA.
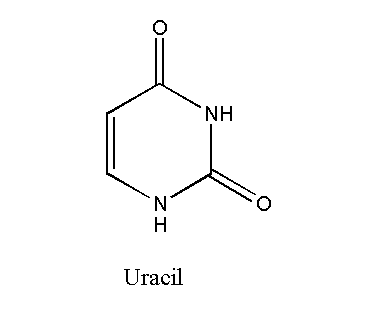
Uracil
Uracil is a pyrimidine with the chemical formula C4H4N2O2. The uracil-containing nucleotide is called uridine. Uracil is basically the demethylated form of thymine. The process of removal of the methyl group from the molecule is called demethylation. Uracil forms a bond with adenine in the RNA. It replaces thymine.
These nitrogenous bases are combined with sugar and phosphate groups to form nucleotides. These nucleotides then combine with each other to form nucleic acids.
Sugar
The second portion of the nucleotide is sugar. It is pentose sugar. There are two types of sugars present in nucleic acids:
- Deoxyribose sugar (present in DNA)
- Ribose sugar (present in RNA)
The difference between DNA and RNA is that DNA (deoxyribonucleic acid) contains deoxyribose sugar while RNA (ribonucleic acid) contains ribose sugar. One hydroxyl group is absent in DNA. The sugar of one nucleotide bonds with the phosphate group of the next nucleotide. Thus, the sugar-phosphate backbone is formed which is very strong. This covalent bond is much stronger than the hydrogen bond between two strands. This bond gives strength and rigidity to the structure.
Phosphate group
The last part of the nucleotide is phosphate. Phosphates are derivatives of phosphoric acid. Free nucleotides contain one or two phosphate groups.
Monophosphate contains one phosphate group while diphosphate contains two phosphate groups.
The nucleotides which are bonded together to form nucleic acids contain three phosphate groups therefore known as triphosphate. Adenosine triphosphate (ATP) contains three phosphate groups. So, it can store a large amount of energy.
The bonds in nucleotides are known as phosphodiester bonds. These bonds are formed between phosphate and sugar.
During the replication of DNA, an enzyme DNA polymerase adds the correct bases and arranges them in a chain. After this, another enzyme DNA ligase makes the phosphodiester bonds between the sugar on one nucleotide and phosphate on the next nucleotide. This forms the backbone which passes to the next generation. DNA and RNA contain all the genetic information necessary for cell function.
Functions of nucleotides
- Nucleotides are the building blocks of nucleic acids (DNA and RNA). These nucleotides contain all the genetic information.
- Free nucleotides act as co-enzymes. A coenzyme is a substance that is produced by a living organism and acts as a catalyst. The co-enzymes are needed for many cellular and biological functions. These co-enzymes are NAD and NADP. NAD and NADP also play role in many redox reactions. They act as electron carriers.
- Nucleotides make the major signaling substance known as cyclic AMP (cAMP). cAMP is a secondary messenger involved in cell signaling. It transports chemical signals between the cells. the cAMP also regulates the metabolism.
- ATP also contains nucleotides. ATP is the main energy storage in the body. When energy is needed in the body, ATP converts into ADP or AMP. ATP also acts as a coenzyme.
- Free nucleotides play important role in cellular metabolism. This is the process in which cellular degradation occurs in the cell and this happens in the DNA and RNA. This cellular degradation occurs due to chemical changes in the nucleotides.
- Nucleotides are not just building blocks of DNA and RNA but also form many molecules that are necessary for the functioning of cells.