LEARNING OBJECTIVES
In this article, you will learn about hydrogen bonding such as types of hydrogen bonding, properties of hydrogen bonding, and applications of hydrogen bonding with examples by a professional author.
Watch the video lecture for a better understanding of Hydrogen bonding
Hydrogen bonding
Table of Contents
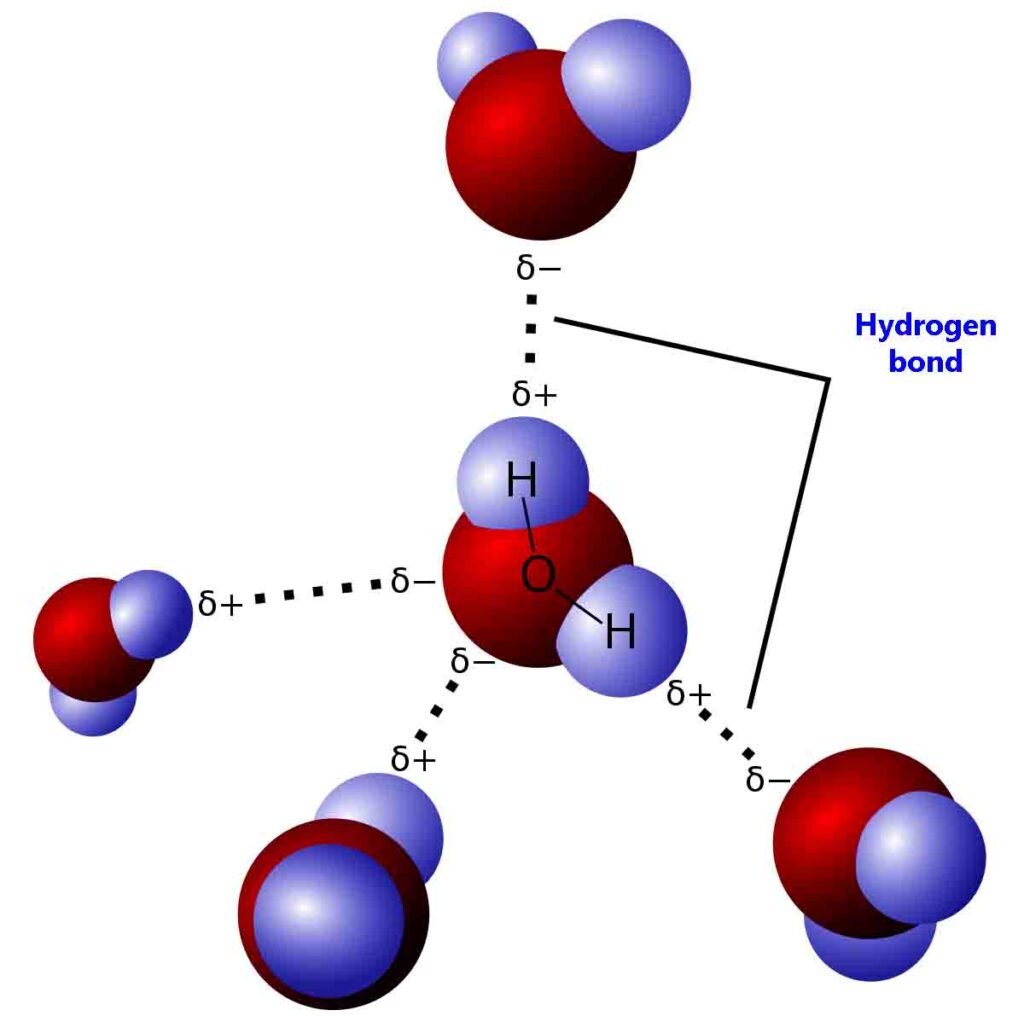
The electrostatic interaction between a strongly electronegative atom and a partially positively charged hydrogen atom is called hydrogen bonding.
Points to be remembered for hydrogen bonding
- The strong electronegative elements are mostly N, O, F and Cl.
- Two molecules involved in Hydrogen bonding may be the same or different.
- Exceptionally low acidic strength of HF molecule as compared to HCl, HBr, and HI is due to strong hydrogen bonding.
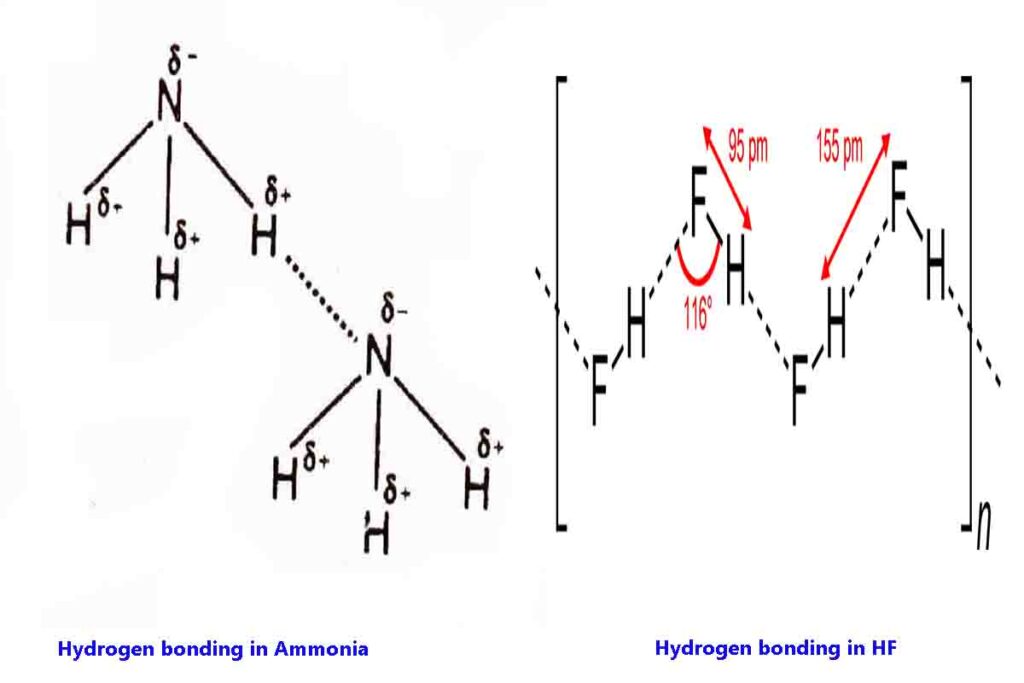
- Ammonia and hydrogen fluoride can form only one hydrogen bond due to the presence of only one utilizable lone pair of electrons and one utilizable H atom respectively.
- Water can form two hydrogen bonds as it has two utilizable hydrogen atoms and utilizable lone pairs on the oxygen atoms.
Hydrogen bonding in different molecules
Hydrogen bonding in water
Consider H2O. in H2O strong electronegative oxygen atom attracts a shared pair of electrons more towards itself. therefore oxygen atom gets a large partial negative charge and the hydrogen atom gets a large partial positive charge.
Thus dipole-dipole interaction may be developed among water molecules. however, forces of attraction among water molecules are stronger than simple dipole-dipole interaction.
The reason is that oxygen has two lone pairs of electrons. moreover, the hydrogen atom creates a strong electric field due to its small size. Therefore the oxygen atom of one water molecule linked to the hydrogen atom of another water molecule true lone pair by a co-ordinate covalent bond. This bond formed is called a hydrogen bond.
Hydrogen bonding in HF and NH3
The hydrogen bond between HF and NH3 molecules are represented as following
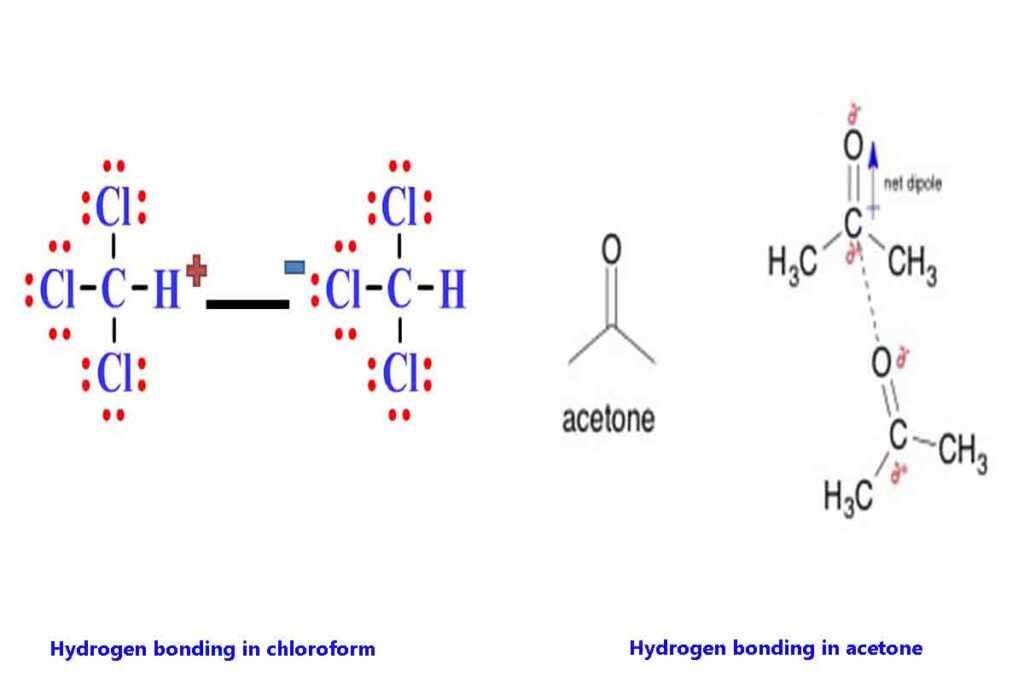
Hydrogen bonding in acetone and chloroform
Some elements other than Nitrogen Oxygen and fluorine are also involved in hydrogen bonding. For example, in chloroform, the three chlorine atoms make the carbon atom highly partial positively charged which in turn makes the hydrogen atom also highly partial positively charged.
This hydrogen atom can now form hydrogen bond with strong electro negative atom of other molecule such as oxygen atom of acetone as shown in the pic.
Properties of hydrogen bond
- The hydrogen bond is longer than a normal covalent bond
- The hydrogen bond is stronger than dipole-dipole interaction but weaker than the normal covalent bond. it is generally 20 times weaker than a covalent bond.
- The hydrogen bond is a directional Bond.
- Hydrogen bond results in the formation of long chains and a network of molecules.
Properties and applications of compound containing hydrogen bonding
Hydrogen bond help in many approaches mentioned below
1: strength of acids
HF is a weaker acid than HCl, HBr, and HI.
In HF molecules are hydrogen-bonded in a zigzag manner. Hydrogen is in a trap between two fluorine atoms as shown in the figure.
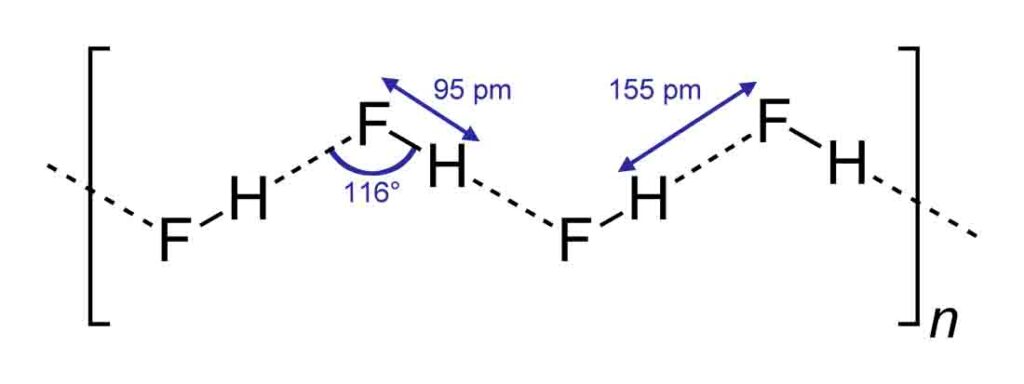
Therefore HF cannot easily donate if H+ ions easily hands it is a weak acid.
2: Thermodynamic properties of covalent hydrides
In covalent hydrides of group IVA to VII A hydrogen bonding effects are very clear.
Consider the graph for boiling point of covalent hydride plotted against their period number.
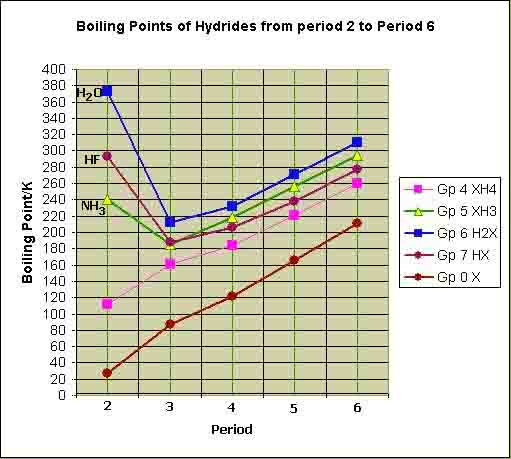
The boiling point of hydrides of group IV A are lowest among all covalent hydride.
It is because elements of group IV A are least electronegative therefore they have weakest intermolecular forces and anger all covalent hydrides. for example, CH4 has lowest boiling point because it is a very small molecule and it has least polarizability.
The boiling point of NH3, HF, and H2O are highest in their respective series.
It is because these hydrides have strongest electronegativity elements such as nitrogen fluorine and oxygen which farm hydrogen bonding among their own molecules. Therefore their boiling points are high.
H2O has high boiling point than HF although fluorine is more electronegative than oxygen.
It is because chlorine atoms can make only one hydrogen bond per molecule due to the presence of one hydrogen atom. While H2O can form two hydrogen bonds per molecule because it has two hydrogen atoms and 2 lone pairs of electrons. Due to strong hydrogen bonding in H2O, its boiling point is greater than HF.
The boiling point of NH3 is lower than HF and H2O
NH3 can someone hydrogen bond for molecules it is because nitrogen has only one lone pair of electrons. Because its electronegativity is lower than oxygen and fluorine. Therefore its boiling point is lower than HF and H2O.
H2O is a liquid but H2S and H2Se are gases
In H2O strong hydrogen bonding is present which makes it a liquid. In H2S and H2Se weak intermolecular forces are present. Therefore H2S and H2Se are gases at room temperature.
The boiling point of HBr is higher than HCl
It is due to the bigger size of bromine than chlorine.
Due to the bigger size of bromine, HBr has greater polarizability and stronger London forces among its molecule than HCl. therefore boiling point of HBr is greater than HCl.
The hydrides of the 4th period, GeH4, AsH3, H2Se, and HBr show greater boiling points than those of the third period due to larger size and greater polarizabilities.
3: Solubility
Both H2O and Ethyl alcohol are highly miscible with each other
Substances which can form H-bond with each other, are highly soluble in each other. Since both H2O and ethyl alcohol can form hydrogen bonding with each other, therefore, these are miscible with each other in all proportions.
However, larger alcohols are not soluble in water due to non-polar nature of bigger hydrocarbon chain in them.
Similarly, small carboxylic acids are also soluble in water.
Hydrocarbons are insoluble in water
It is because these are non-polar and cannot develop H-bonding or other attractions with H2O. Hence hydrocarbons are insoluble in water.
4: Cleansing action
Soaps and detergents are made up of long non-polar hydrocarbons (alkyl or benzyl) and a polar anion head. In water, the head is stabilized by making H-bond with H2O while the non-polar tail remains outside H2O because it is not soluble in water.
Therefore, hydrogen bonding helps in cleansing action.
5: Hydrogen bonding in biological compounds and food material
Hydrogen bonding is very important in living organisms.
Large protein molecules in living organisms are stabilized due to H-bonding
Many fibrous proteins such as horn, nail, skin, feather, hair etc. are composed of long chains of amino acids. These chains are coiled around each other and form a spiral structure. This spiral is called helix.
Such helix may either be right handed or left handed. In right handed helix groups like NH and C=O are vertically adjacent to one another and these form H-bonds. These hydrogen bonds link one spiral to the other.
X-ray analysis have shown that on the average there are 3.6 amino acids for each turn of the helix.
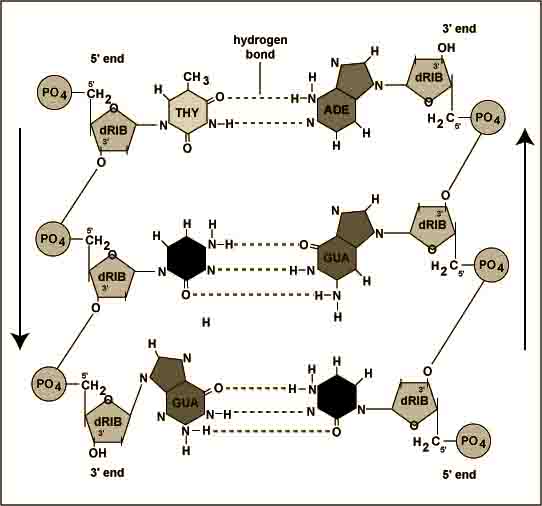
DNA( deoxyribonucleic acid) occurs in cells
It consists of two spiral chains which are coiled about each other on a common axis and form double helix. This is 18-20 A0 in diameter. These are linked together by hydrogen bonding between their subunits.
6: Hydrogen bonding in paints and Dues
The adhesive nature of certain paints and dyes is also due to H-bonding with the surfaces.
Similarly, sticky action of glue and Honey is also due to H-bonding.
7: Hydrogen bonding in food material
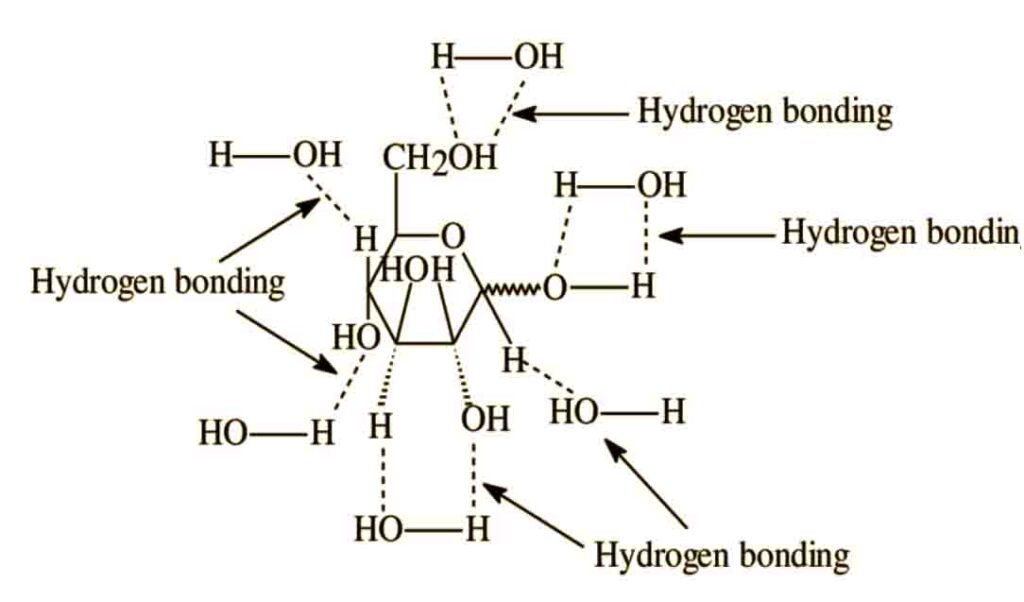
Food material such as carbohydrates such as glucose, fructose and sucrose are also stabilized due to H-bonding. All these contains OH group which produce H-bonding.
8: Hydrogen bonding in clothing
Both natural and artificial fivers have rigidity and tensile strength due to H-bonding.
9: Hydrogen bonding in the structure of ice
H2O has a tetrahedral electronic structure.
Ice floats on water
In liquid H2O, molecules form a temporary H-bond with each other. It is due to the movement of molecules, bonds are broken and reformed. Therefore, there is less regularity and less free space.
However, when the temperature of H2O is lowered below 40C, its molecules become regular and form permanent H-bond. So empty spaces are developed in between the molecules and their volume increases.
This is the reason that ice occupies 9% more space than liquid water. Thus the density of ice becomes less than water. Hence it floats over water.
Similarity between the structure of ice and diamond
The structure of ice is just like that of a diamond because each atom of carbon in the diamond is at the center of the tetrahedron just like the oxygen of water molecule in ice. It has a hexagonal structure with large empty spaces.
Applications of low density of ice in clod climates
Density of ice is less than water. Therefore, in clod climate, when temperature falls below 40C, clod water being lighter comes to the surface and freezes to ice.
Thus an insulating layer of ice is formed above warm water. This layer of ice prevents further heat loss from underneath water. Therefore, it protects aquatic life from cold.