LEARNING OBJECTIVES
In this article, author has explained structure, physical and chemical properties, uses, benefits and drawbacks of sucrose.
Sucrose is a carbohydrate composed of monosaccharides; glucose and fructose. Sucrose is a non-reducing disaccharide which means it does not have a free aldehyde group. The common name of sucrose is “table sugar”. It is also known as “cane sugar”.
Sucrose is a sweetener that is derived from sugarcane. It is a carbohydrate that is added to baked goods and candies. In fact, it is the main ingredient in marshmallows and ice cream.
The most important thing is that it has a low glycemic index. This means that it is absorbed by the body slowly and it is not digested as quickly as other sugars.
But there is another side of sucrose. Yes, it is a sweetener, but it has a number of health benefits.
Sucrose is the least toxic compared to other sugars and it contains zero cholesterol.
It is also an excellent antioxidant. It reduces the risk of heart disease and stroke by improving blood circulation and lowering bad cholesterol. It has also been found that it has a protective role against cancer and diabetes.
Sucrose is the sweetest among all the carbohydrates. It is also produced by sugar cane and sugar beet. The enzyme “sucrase” present in the intestine breaks sucrose into glucose and fructose which are then absorbed in the intestine.
Sucrose is primarily derived from sugar cane, sugar beets and corn. It is also sometimes produced from sugar beet molasses. It is a naturally occurring compound that is found in some fruits, and in various types of honey.
Structure of sucrose
Table of Contents
Sucrose is formed by the combination of two monosaccharides. The glycosidic bond is formed between the C1 of α-D-glucose and the C2 of β-D-fructose.
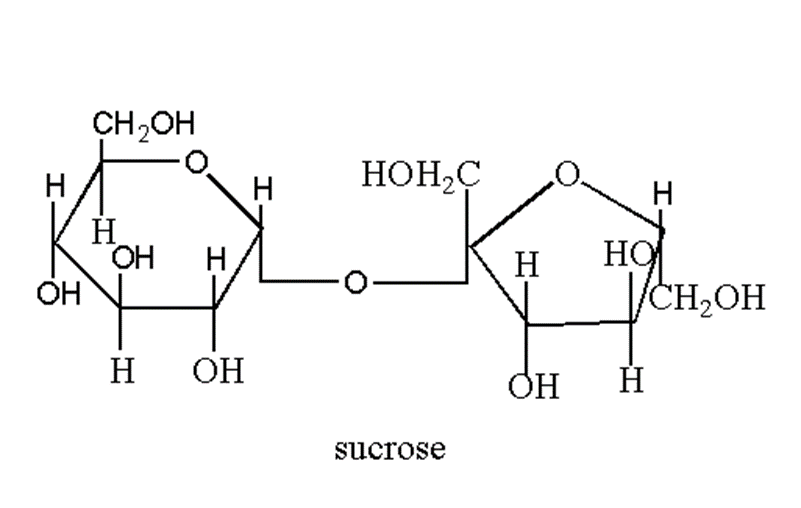
So as clear from the structure, sucrose is made of the glycosidic linkage between C1 of alpha glucose and C2 of beta fructose. The reducing groups of sucrose are involved in a glycosidic bond that’s why it is a non-reducing sugar.
Physical Properties of sucrose
- Sucrose is a non-reducing sugar.
- It appears as a white crystalline solid.
- The molecular formula of sucrose is C12H22O11.
- The mass of sucrose is 342.30g/mol.
- Its density is 1.587g/cm3.
- Sucrose is the sweetest among all other carbohydrates.
- It is readily soluble in water.
- Under high temperatures, sucrose decomposes.
Chemical properties of sucrose
- When treated with sulfuric acid, sucrose gets dehydrated.
C12H22O11 + H2SO4 —> 11H2O + 12C + heat
- On treatment with hydrochloric acid, sucrose undergoes combustion.
C12H22O11 + 8HClO3 —> 8HCl + 11H2O + 12CO2
- On hydrolysis, sucrose is broken into glucose and fructose.
Applications of sucrose
Sucrose is the sweetener that is used to make confectioneries, baked goods, candies, jellies, cakes, frosting, yogurt, sauces, ice cream, pies, puddings, beverages, and many more. There are a lot of applications of this sweetener, but these are the most common uses.
For baking
You can use this sweetener to make frosting and to make a variety of cakes. It is available in a lot of variations, so choose the right one depending upon the taste and texture that you want to make.
For beverages
It is the best sweetener for hot chocolate, iced tea, lemonade, coffee, and many more. You can use it in any of the drinks that you like.
For candy making
It is the best sweetener for making candies and sugar coating.
For jelly making
Jelly is the best sweetener that can be used to make fruit jellies and jams. You can add it to any kind of jelly that you want.
For icing
This is the best sweetener for cakes, cookies, cupcakes, and other desserts. It is available in a variety of flavors and colors.
For making sorbet
It is the best sweetener for sorbets, ice creams, and frozen treats.
For topping
This is the best sweetener for toppings, frostings, and toppings.
For yogurt
This is the best sweetener for yogurts, kefir, and fermented drinks.
Advantages of sucrose
- Sucrose stores a large amount of energy.
- It is the most important carbohydrate which is used as an energy source.
- Sucrose has a major advantage in food industries where it is frequently used for its sweet taste.
- It provides the energy which is necessary for the physical and mental functions of the body.
- Sucrose is naturally found in many fruits, vegetables, and plants from where it can be extracted and used as a sweetening and flavoring agent in food industries.
Disadvantages of sucrose
- Consuming too much sucrose can leads to diabetes.
- Sucrose can cause tooth decay and other mouth diseases.
- Sucrose contains a large amount of glucose and fructose. When sucrose breakdowns in boys, it can raise their blood sugar level.
- It can cause an increase in body weight.
- It can lead to a heart problem.
- Sucrose can cause obesity as it is a sweetest carbohydrate.
- In short, taking too much sucrose can cause several health problems.