LEARNING OBJECTIVES
In this article, author has explained introduction, occurrence, structure, physical and chemical properties, classification and functions of triacylglycerols.
Definition
Table of Contents
The esters of glycerol with fatty acids are called triacylglycerols. Triacylglycerols are widely distributed in plants and animals. They are also known as neutral fats. The common name of triacylglycerols is triglycerides. Oxidation of triacylglycerols provides the twice energy as compared to that of polysaccharides. In many organisms, extra carbohydrates are also stored in the form of triglycerides.
Occurrence
Triacylglycerols are stored in the adipocytes cells of adipose tissues. Adipose tissue is found in the subcutaneous layer and abdominal cavity. Triacylglycerols are stored in the form of globules present in the entire cytoplasm. Triacylglycerols are not a component of biological membranes.
Structure of triacylglycerol
Triacylglycerols consist of three fatty acids esterified to each carbon of the glycerol molecule.
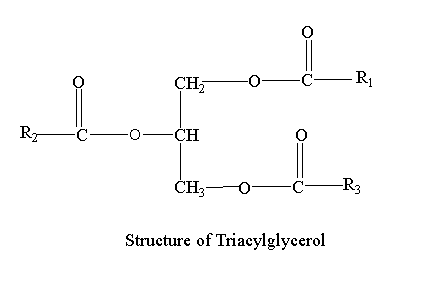
Three hydroxyls (OH) groups react of single glycerol react with carboxyl (COOH) groups of three fatty acid chains. Glycerol is simple alcohol with three hydroxyl groups available. As a result ester bond is formed and a triglyceride is generated. When energy is demanded, stored triacylglycerol is digested and unsaturated fatty acids are released into the circulatory system and delivered to tissues.
Physical properties of triacylglycerols
- Triacylglycerols are the simplest lipids made up of fatty acids.
- They are widely distributed in plants and animals.
- They are non-polar.
- Hydrophobic in nature
- Insoluble in water.
- Soluble in organic solvents.
- Their specific gravity is less than water therefore fats and oils float on water.
- The melting point of saturated triacylglycerols is higher than unsaturated.
Chemical properties of triacylglycerols
1. Hydrolysis
Triacylglycerols undergo hydrolysis to yield fatty acids and glycerol. The hydrolysis reaction is catalyzed by the enzymes known as lipases. Lipases are important for the digestion of fats in the gastrointestinal tract and fat mobilization from the adipose tissues.
2. Saponification
Hydrolysis of triacylglycerols using alkali is called saponification. This reaction produces glycerol and soaps.
Triacylglycerol + 3NaOH—> Glycerol + 3R-COONa (soap)
3. Rancidity
The term rancidity refers to the deterioration of fats and oils. It causes an unpleasant taste and odor. Fats that contain unsaturated fatty acids have more chances to undergo rancidity. Rancidity occurs when fats are exposed to light, moisture, and bacteria.
Hydrolytic rancidity occurs due to partial hydrolysis of triacylglycerols by bacterial enzymes.
Oxidative rancidity occurs due to the oxidation of unsaturated fatty acids. This results in the formation of dicarboxylic acids, aldehydes, ketones, etc. These are unpleasant products. Rancid fats and oils are not suitable products for humans to consume.
The substances which prevent the oxidation of unsaturated fatty acids are called antioxidants. For example, tocopherols, hydroquinone, gallic acid, and D-naphthol are some antioxidants that are added to fats and oils to prevent rancidity during the preparation of fats and oils. Propyl gallate, butylated hydroxyanisole, and butylated hydroxyl toluene are antioxidants used in the preservation of food.
4. Lipid peroxidation in vivo
Lipids undergo oxidation in the living cells. As a result, peroxides and free radicals are produced. They can damage tissues. The free radicals cause inflammatory diseases such as aging, cancer, and atherosclerosis. Cells have some antioxidants such as vitamin E, urate, and superoxide dismutase. These antioxidants prevent in vivo peroxidation of lipids.
Classification of triacylglycerols
The classification of triacylglycerols is based upon the type of fatty acid present.
Saturated triacylglycerols
The triglycerides which contain saturated fatty acids (no double bond) are called saturated triglycerides. Saturated triglycerides are the main reason for the high LDL and cholesterol. Saturated fats are found in many things like butter, cheese, milk, meat, etc.
Monounsaturated triacylglycerols
These triglycerides have monounsaturated (one double bond) fatty acids. Monounsaturated fatty acids as compared to saturated fatty acids lower the level of LDL and cholesterol. Monounsaturated triglycerides are present in olive oil, canola oil, and peanut oil.
Polyunsaturated triacylglycerols
These triglycerides have polyunsaturated (two or more than two double bonds) fatty acids. They also help to lower cholesterol levels. They can be found in vegetable oils and many fishes like salmon, trout, and mackerel.
Triacylglycerols of plants have a higher content of unsaturated fatty acids as compared to that of animals.
Simple triacylglycerols
The triacylglycerols which have the same type of fatty acids as all the three carbon atoms are called simple triacylglycerols.
For example, tristearoyl glycerol or tristearin
Mixed triacylglycerols
These triacylglycerols have two or three types of fatty acids. The saturated fatty acid is present on carbon 1, and unsaturated fatty acid is present on carbon 2 while on carbon 3 can be either.
1,3-palmitoyl 2-linoleoyl glycerol is a mixed triacylglycerol.
Functions of triacylglycerols
- The main function of triacylglycerol is to store energy in the body. When we take a diet, it provides energy to the body, and the excess is stored in the form of triacylglycerols in the adipose tissues. During fasting conditions, the stored triacylglycerols are digested and fatty acids released. Hormones signal to release the triglycerides. This provides the necessary calories to the body.
- Triglycerides are the most abundant fuel reserve for animals. The fat reserve of a normal human is sufficient to meet the body’s caloric requirement for 2-3 months.
- Triglycerides are necessary for the proper functioning of the body. Without triglycerides, the body will run out of energy.
- Triglycerides are a better way to store energy than carbohydrates because the oxidation of triglycerides provides the double amount of energy as the oxidation of carbohydrates.
- Triglycerides under the skin provide insulation against the changing temperature.