Written By Adeel Abbas
Definition
Table of Contents
Iodometric titration is a method of quantitative analysis that involves the indirect determination of the concentration of an oxidizing agent in a sample solution.
This redox titration relies on the reaction between the oxidizing agent and iodide ions to produce iodine, which is then titrated using a standardized sodium thiosulfate solution.
The endpoint of the titration is indicated by the disappearance of the deep blue color of the starch-iodine complex, offering high precision in determining analyte concentration.
In our previous discussions, we have covered the broader concept of titration, which encompasses various types such as acid-base, redox, complexometric, and precipitation titrations. Each type of titration serves a specific purpose in quantitative analysis, allowing us to determine the concentration of analytes in a wide range of samples.
Moreover, we have explored the uses of titration in diverse fields, ranging from pharmaceuticals to environmental monitoring. This powerful analytical tool enables scientists to measure the concentration of substances with accuracy and reliability, providing valuable insights into chemical processes and ensuring quality control in various industries.
In the titration, the choice of indicators plays a crucial role in determining the endpoint of the titration reaction. We have delved into the fascinating world of indicators, both natural and synthetic, which serve as vital tools in visualizing the completion of titration reactions.
Natural indicators, such as litmus, turmeric, and red cabbage extract, harness the inherent color-changing properties of organic compounds. On the other hand, synthetic indicators, such as phenolphthalein and methyl orange, are carefully synthesized compounds specifically designed for titrimetric applications.
Now, let us delve into the intricacies of iodometric titration and discover its principles, procedure, advantages, and practical applications.
Principle of Iodometric Titration
Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. To know when the reaction is complete, we use starch solution as an indicator. The starch forms a blue color when it reacts with iodine. We can tell that the reaction is finished when the blue color fades away.
Procedure of Iodometric Titration
To perform an iodometric titration, we need to follow a step-by-step process carefully. First, we dissolve an oxidizing substance in a suitable liquid. Then, we add sulfuric acid, hydrochloric acid, or acetic acid to make the solution acidic. This acid helps the next part of the reaction. Next, we introduce chlorine, which causes the release of iodide ions.
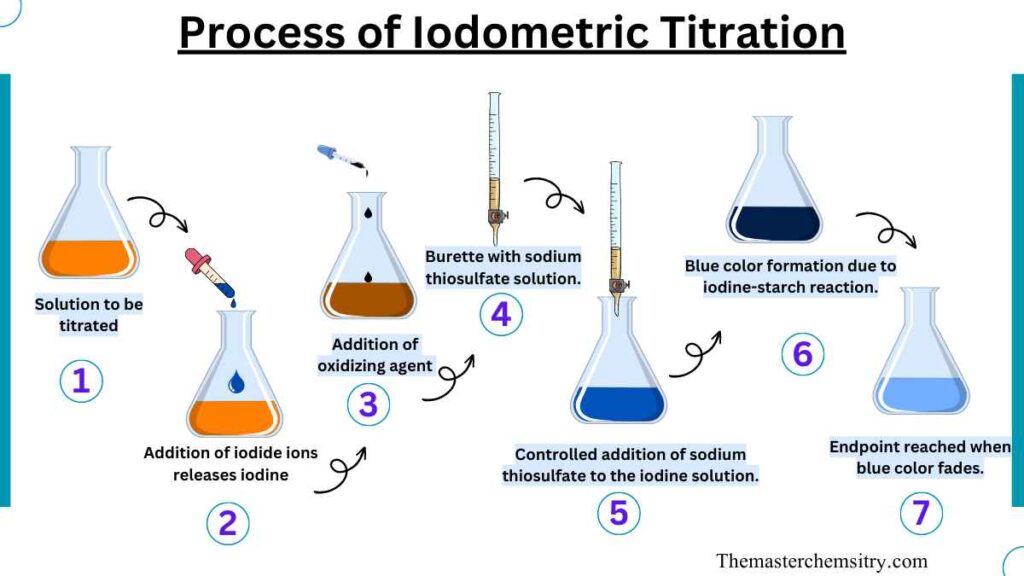
The freed iodide ions are then titrated using a standardized solution of sodium thiosulfate. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. This change in color tells us that the titration is complete.
Advantages of Iodometric Titration
Iodometric titration offers several noteworthy advantages, solidifying its position as a valuable analytical technique. Firstly, it allows for the precise determination of both reducing and oxidizing agents, expanding its analytical versatility. The visible color change associated with the formation of the starch-iodine complex provides a convenient visual indicator for identifying the endpoint of the titration.
Additionally, iodometric titration requires only small quantities of chemicals or substances, making it a cost-effective and efficient analytical method. The presence of iodine in starch imparts a vivid blue color change, facilitating the easy detection of the completion of the reaction. Moreover, iodometric titration offers the opportunity to visually observe reactivity at equilibrium points, fostering a deeper understanding of redox chemistry and its applications.
Applications of Iodometric Titration
Let us explore some practical applications of iodometric titration, demonstrating its relevance in analytical chemistry. One notable example involves the standardization of sodium thiosulfate (Na2S2O3) using potassium dichromate (K2Cr2O7). By accurately determining the concentration of the sodium thiosulfate solution, we can precisely quantify the concentration of the potassium dichromate, a powerful oxidizing agent.
Another application of iodometric titration involves the estimation of Cu(II) (copper(II) oxide) using a sodium thiosulfate solution. This method enables the determination of copper(II) oxide concentration in a given sample, contributing to industries reliant on copper-based materials.
Furthermore, iodometric titration finds application in the estimation of vitamin C, a potent reducing agent, through the iodometric method. This allows for the accurate quantification of vitamin C concentration in various biological samples, shedding light on its role in human health and nutrition.
The realm of iodometric titration unfolds as a captivating avenue in analytical chemistry, empowering scientists to unravel the mysteries of oxidizing agents’ concentrations. With a comprehensive understanding of its principles, procedure, advantages, and practical applications, we equip ourselves with the tools to navigate the intricate world of redox analysis.