Learning objectives
Table of Contents
In this article, the author has explained Acid base titration, its working principle types and process of acid base titration
Acid-base reactions are used to find the amount of acidic or basic substances. The solution with unknown molarity is the analyte. The analyte will react with the solution with known molarity.
First lets start with a simple definition of titration.
What is titration?
Titration is a chemical analysis method used to determine the concentration of a particular analyte.
Titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and an indicator is usually employed to mark the endpoint of the reaction.
Theory of titration
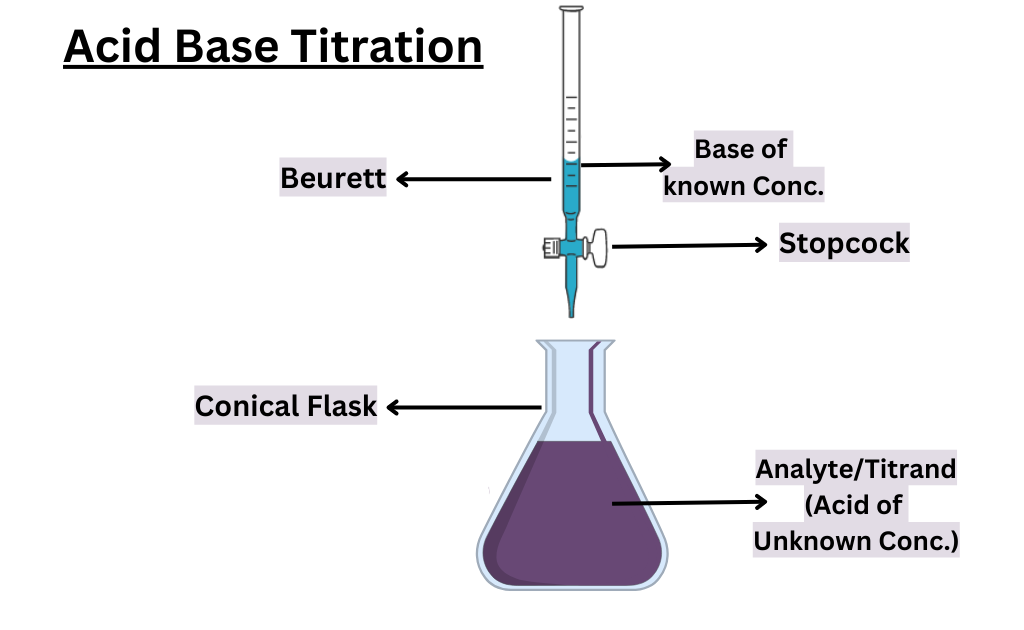
Titration is a method used to determine the unknown concentration of a substance in a solution. It involves the gradual addition of a known concentration of a solution, called the titrant, to a known volume of the substance being analyzed, called the analyte or titrand. The point at which the two solutions are chemically balanced is called the endpoint of the titration.
Types of titration
There are several types of titrations, including acid-base titrations, redox titrations, and complexometric titrations. Acid-base titrations involve the reaction between an acid and a base to form a salt and water. Redox titrations involve the transfer of electrons from one species to another. Complexometric titrations involve the formation of a complex between the analyte and the titrant.
How endpoint of titration is measured?
The endpoint of the titration can be determined using a variety of methods, including visual indicators, conductivity measurements, or pH measurements. The endpoint is typically indicated by a change in the color of the solution, a change in the electrical conductivity of the solution, or a change in the pH of the solution.
Titrations are widely used in various fields, including chemistry, biology, and environmental science, to determine the concentration of a variety of substances in solutions.
important terms in titration
| Term | Definition |
|---|---|
| Analyte | The substance whose concentration is being determined in a titration. |
| Titrant | The solution of known concentration that is added to the analyte in a titration. |
| Endpoint | The point at which the chemical reactions between the analyte and the titrant are balanced. This is usually indicated by a change in the color of the solution, a change in the electrical conductivity of the solution, or a change in the pH of the solution. |
| Equivalence point | The point at which the number of moles of the titrant added to the analyte is equal to the number of moles of the analyte present. At this point, the volume of titrant added can be used to calculate the concentration of the analyte. |
| Burette | A piece of laboratory equipment used to measure and dispense precise volumes of a solution. The volume of titrant added to the analyte can be determined by reading the volume on the burette at the beginning and end of the titration. |
| Titration curve | A graph of the volume of titrant added versus the concentration of the analyte. The titration curve typically has a distinct inflection point at the equivalence point, which can be used to determine the concentration of the analyte. |
| Indicators | Substances that are used to indicate the endpoint of a titration. Indicators are typically chosen based on their ability to undergo a visible color change at a specific pH. |
I have also written few titration related other articles, I am sure you would learn from these as well.
- What are precautions during titration process in lab
- Uses of titration in industries
- Why is titration important in chemistry?
What is acid-base titration?
An acid-base titration is a quantitative analysis method used to determine the concentration of an acid or base by neutralizing the acid or base with a known concentration standard solution.
The concentration of a solution can be determined by knowing the acid and base dissociation constant. If the solution concentration is known, a titration curve can be used.
Principle of acid-base titration
In the theory of acid-base titration, the principle involves using a burette and pipette to determine the concentration of an acid or basic.
An indicator is a dye added to a solution to change its color. It is dissolved in the sample solution and can be used to detect the end of the titration.
Importance of acid base titration
Acid-base titrations are important because they allow for the precise determination of the concentration of an acid or a base in a solution. This information is useful in a variety of fields, including chemistry, biology, and environmental science.
In chemistry, acid-base titrations are used to determine the concentration of acids and bases in solutions, which is important for understanding chemical reactions and for the preparation of standard solutions.
In biology, acid-base titrations are used to determine the pH of solutions, which is important for understanding the behavior of enzymes and other biological molecules.
In environmental science, acid-base titrations are used to determine the acidity or basicity of water, which is important for understanding the impact of acid rain on aquatic ecosystems.
Overall, acid-base titrations are a powerful tool for understanding and quantifying the concentration of acids and bases in solutions, and they have many important applications in various fields.
Acid base titration and volumetric analysis
Acid-base titrations are a type of volumetric analysis, which is a method of chemical analysis that involves the measurement of volume in order to determine the concentration of a substance in a solution. In an acid-base titration, a solution of known concentration (called the titrant) is gradually added to a known volume of the substance being analyzed (called the analyte). The point at which the two solutions are chemically balanced is called the endpoint of the titration.
Indicators used in acid-base titration:
The acid strength of the indicator is important in determining the pH range. The indicator changes color from acid to base when it’s in the range of pH values.
The acid form can only be seen at the highest pH, the base form can only be seen at the lowest. Since the indicator doesn’t change color at certain pH levels, it’s not sensitive to changes outside of its range.
Selection of indicator in acid base titration
There are several factors to consider when selecting an indicator for an acid-base titration:
- The pH range of the titration: The indicator should have a color change within the pH range of the titration. For example, if the titration involves a strong acid and a strong base, the pH range will be wide, and an indicator with a wide range, such as bromothymol blue, can be used. If the titration involves a weak acid and a strong base, the pH range will be narrow, and an indicator with a narrow range, such as phenolphthalein, can be used.
- The desired endpoint: The indicator should undergo a color change at the desired endpoint of the titration. For example, if the endpoint of the titration is the point at which the acid and base are neutralized, an indicator with a pK value close to 7, such as bromocresol green, can be used. If the endpoint of the titration is the point at which the acid and base are in a specific ratio, an indicator with a pK value close to the desired ratio, such as methyl red, can be used.
- The sensitivity of the indicator: The indicator should undergo a noticeable color change at the endpoint of the titration. Some indicators, such as thymol blue, have a sharp color change at the endpoint, while others, such as methyl orange, have a more gradual color change.
Overall, the selection of an indicator for an acid-base titration is based on the pH range of the titration, the desired endpoint, and the sensitivity of the indicator.
Classification of acid-base titration indicators
Acid-base indicators are generally classified into below listed three groups.
1. The phthaleins and sulphophthaleins: example-phenolphthalein indicator
2. Azo indicators: example- methyl orange indicator
3. Triphenylmethane indicators: for example- malachite green indicator
Specific indicator for different acid-base titration:
Different indicators are used in acid-base titrations. The selection of indicators depends on the type of titration and the range of the reaction.
- Strong acid-strong base: Phenolphthalein is generally preferred due to color change seen more easily.
- Weak acid-strong base: Phenolphthalein is more proffered for this titration because it changes sharply at the equivalence point.
- Strong acid-weak base: Methyl orange is more proffered for this titration because it changes sharply at the equivalence point.
- Weak acid-weak base: Because a vertical portion of the curve above two pH units is required, there is no indication is suitable for this titration.
Types of acid-base titration with their examples:
There are four different types of acid-base titration, which include strong acid-strong base, weak acid-strong base, strong acid-weak base, and weak acid-weak base.
Strong acid-strong base:
It’s one of the easiest titrations to perform among the four forms of acid-base titrations. It involves the dissociation of a strong acid and a strong base in water, which results in a strong acid-strong base neutralization reaction. The equivalency point is reached when the moles of acid and base are the same and the pH is zero.
Weak acid-strong base
The direct transfer of the protons from the weak acid to the hydroxide ion is possible in this type of titration. The acid and base react in a one-to-one ratio when reacting a weak acid with a strong base. At the equivalent point of a weak acid–strong base titration, the pH is larger than 07.
Strong acid-weak base
The acid and base will react to form an acidic solution. A conjugate acid is formed which reacts with water to form a hydronium ion. At the point of a strong acid-weak base titration, the pH is less than 7.
Weak acid-weak base
The shape of a weak acid’s or base’s titration curve depends on the acid’s or base’s identity and the associated acid ionization constant. In the titration of a weak acid or a weak base, the pH gradually changes around the equivalence point, which is greater or less than 7.
Below are examples of these titrations.
- Hydrochloric acid (HCl) and sulphuric acid (H2SO4) are two examples of strong acids.
- Acetic acid (CH3COOH) and formic acid (CH2O2) are two examples of weak acids.
- Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are two examples of strong bases.
- Ammonia and methylamine are two examples of weak bases.
Apparatus and Process of titration
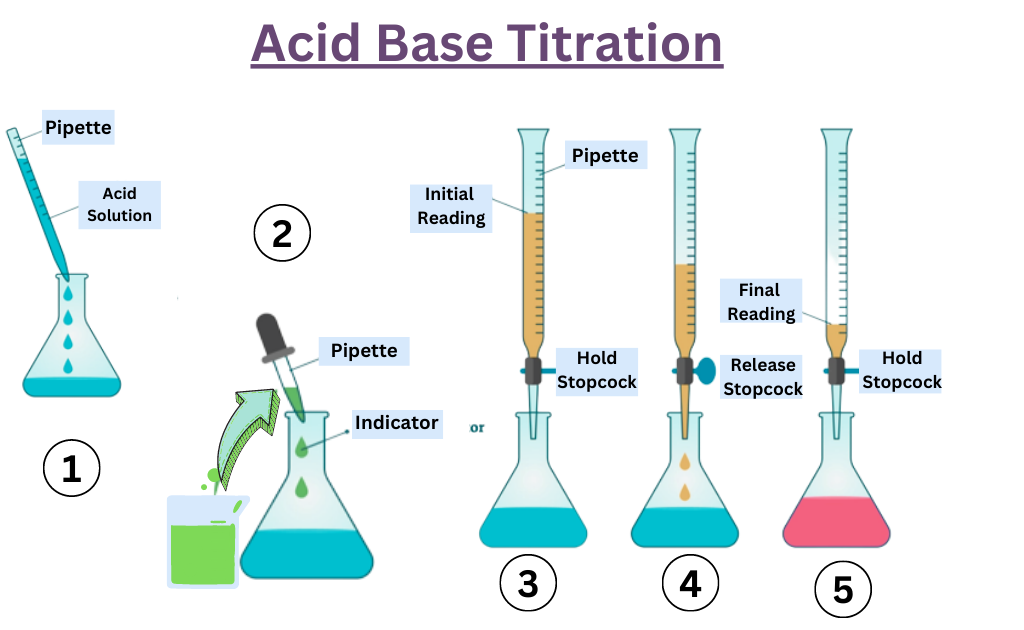
First of all we need to arrange the following apparatus to perform acid-base titration.
- Conical flask
- funnel
- beaker
- pipette
- burette
- burette stand
- Spatula
- wash bottle
- indicator
- unknown solution
- Standard solution
Then we need to clean all the apparatus using distilled water.
After then we need to fill the burette with a standardized solution, accurately measure the volume of the analyte, and add in the conical flask, also add a few drops of indicator using the pipette.
Titrate it with the standardized solution until the indicator changes color. When the indicator permanently changes the color, the endpoint reaches.
Repeat the titration at least three more times and record the initial and final readings in the observation table and calculate the value.
How does titration work?
Titration is a method used to determine the concentration of a substance in a solution. It involves adding a known concentration of a solution (titrant) to a known volume of the substance (analyte) and measuring the volume of titrant at the point of chemical balance (endpoint).
What is the major purpose of acid base titration?
The major purpose of acid-base titration is to determine the concentration of an acid or a base in a solution.
Which indicator is used in acid base titration?
An indicator is chosen for an acid-base titration based on the pH range of the titration, the desired endpoint, and the sensitivity of the indicator. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange.
is titration only for acids and bases?
No, titration can be used to determine the concentration of various substances, including acids, bases, and other types of molecules. There are several types of titrations, including acid-base, redox, and complexometric.
How do acid base indicators work?
Acid-base indicators change color at a specific pH and are used to indicate the endpoint of a titration. They are chosen based on the desired endpoint and the pH range of the titration, and their color change at the endpoint is used to determine the concentration of the acid or base in the solution.
Why is phenolphthalein an appropriate indicator for a weak acid-strong base titration?
Phenolphthalein is a suitable indicator for a weak acid-strong base titration because it has a narrow pH range and undergoes a sharp color change at the endpoint.