LEARNING OBJECTIVES
In this article, the author has explained ionic bonding, conditions favorable for the formation of ionic bond, and factors affecting ionic bond formation. The author has also explained the properties of ionic compounds and examples of ionic compounds.
Definition
Table of Contents
The bond formed by the complete transfer of electrons from one atom to another is called an ionic bond.
Example
Let’s discuss the general example of two atoms A and B. Atom A has one electron while atom B has 7 electrons. Atom B needed 1 electron to complete its octet and to get stable. Atom A has one electron in excess. So, A transfers the electron to B. Both the atoms get stable.
When A loses an electron, it becomes a positive ion (cation) and when B accepts an electron it becomes a negative ion (anion). The opposite charges ions are held together by electrostatic attraction. This type of chemical bonding is known as ionic bonding.
Conditions for the formation of ionic bond
There are conditions that are favorable for the formation of an ionic bond:
1. Number of valence electrons
The atom which is losing electrons must have 1, 2, and 3 electrons. If electrons are above 3, the atom cannot lose it. Similarly, the second atom must have 5, 6, or 7 electrons. Groups, 2 and 3 have one IA, IIA, and IIIA valence electrons respectively. Group VA, VIA, and VIIA have 5, 6, and 7 valence electrons respectively. So, an ionic bond is formed between groups 1, 2, 3, and 5, 6, and 7 elements.
2. Low ionization and high electron affinity
For the formation of an ionic bond, net energy must be low. In other words, energy must be released during the formation of an ionic bond.
Atom A needs the energy to release the electron. This energy is called ionization energy. The ionization energy should be low.
When an electron is added to atom B, it releases energy. This energy is called electron affinity. The electron affinity should be high.
The ionic bond is formed between the atom having low ionization energy and the atom with high electron affinity. For example, an ionic bond is formed between sodium and chlorine.
Na —> Na+ + e– -119kcal
Cl + e– —> Cl– +85kcal
3. Electronegativity difference
The ionic bond is formed if there is a great electronegativity difference between the two atoms. If there is the electronegativity of 2.0 or greater than 2.0 is present then the ionic bond is formed otherwise not.
Sodium has an electronegativity of 0.9 while chlorine has 3.1. Thus electronegativity difference of 2.1 is present between Na and Cl. So ionic bond is formed between sodium and chlorine.
Factors affecting the formation of ionic bond
1. Ionization energy
The metal which is losing electrons should have low ionization energy. The lower the ionization energy, the easier the metal can lose the electron. Hence ionic bonds can form easily.
This is the reason that alkali metals and alkaline metals form the ionic bond easily. The ionization energy decrease as we move down the group. So the elements present at the bottom can form an ionic bond very easily.
2. Electron affinity
The atom which is accepting the electron and becoming a negative ion should have high electron affinity. The higher the electron affinity, the greater will be the energy release and the anion will be stable.
The elements of group VIA and VIIA have high electron affinity and thus can form ionic bonds easily. When we move down to the group, electron affinity decreases, and the tendency to form ionic bonds also decreases.
3. Lattice energy
The amount of energy released when one mole of the ionic compound is formed by the combination of anions and cations is called lattice energy.
A+ + B– —> AB + Lattice energy
After the formation of anion and cation, they combine to form an ionic bond. The energy released during the process is called lattice energy. The greater the lattice energy, the greater will be the strength of the ionic bond. The lattice energy depends upon two factors:
a. Size of ion
The smaller the size of the cation and anion, the greater will be the force of attraction between them. The force of attraction is inversely proportional to the size of the atom.
b. Charge on ions
The greater the charge on the anion and cation, the greater will be the force of attraction between them. The strength of the ionic bond will also be greater.
Examples of an ionic compound
Here are some examples of compounds containing ionic bonds:
Sodium chloride (NaCl)
The electronic configuration of sodium is 1s2, 2s2, 2p6, 3s1 and that of chlorine is 1s2, 2s2, 2p6, 3s2,, 3p5. Na transfers its one valence electron to the chlorine.
Na —> Na+ + e–
Cl + e– —> Cl–
In this way, the octet of both Na+ and Cl– is stabilized and an ionic bond is formed between them.
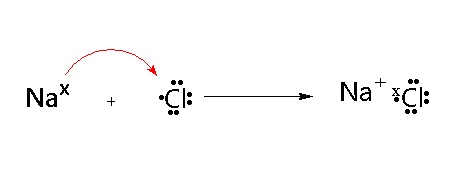
Magnesium Chloride (MgCl2)
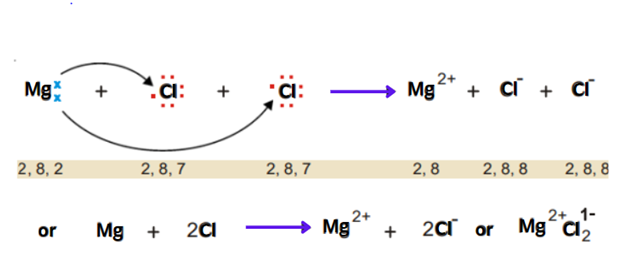
Magnesium has two electrons in its valence shell. Chlorine has seven electrons in its outer shell. Magnesium loses two electrons and transfers one electron to each of one chlorine atom. Thus an ionic bond is formed.
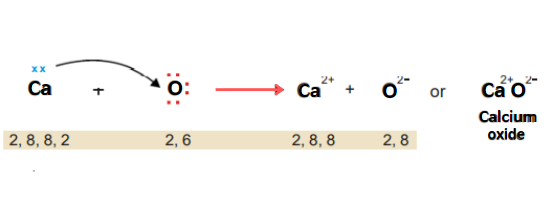
Calcium oxide (CaO)
Calcium has two electrons in its valence shell. Oxygen has 6 valence electrons. Thus calcium transfers its two electrons to the oxygen atom. In this way, both calcium and oxygen achieve a stable electronic configuration and an ionic bond is formed.
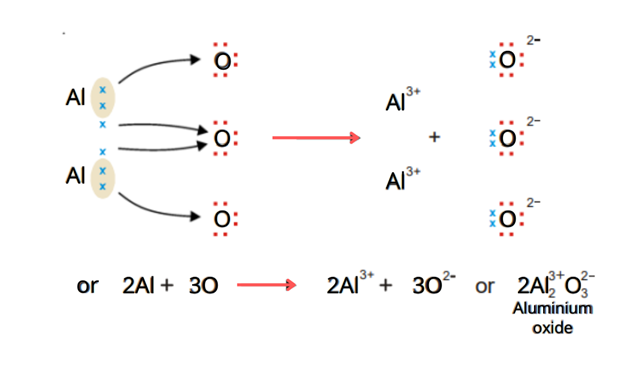
Aluminum Oxide (Al2O3)
Aluminum has three valence electrons while oxygen has 6 electrons. The two aluminum atoms have total 6 six electrons. They transfer two electrons to each oxygen atom. In this way, the stable octet of both aluminum and oxygen is achieved.
The ionic bond is formed between 2Al3+ and 3O2-.
Properties of ionic compounds
1. Appearance at room temperature
An ionic bond is formed by electrostatic attraction between oppositely charged ions. There is a strong force of attraction due to which these ions cannot move from their position. Therefore, ionic compounds are solid at room temperature.
2. Melting points
Due to the strong force of attraction between anions and cations, they cannot easily move. Therefore, the melting points of ionic compounds are high. At sufficiently high temperatures, ions acquire kinetic energy that overcomes the attractive force. So only at high-temperature ions can move from their positions.
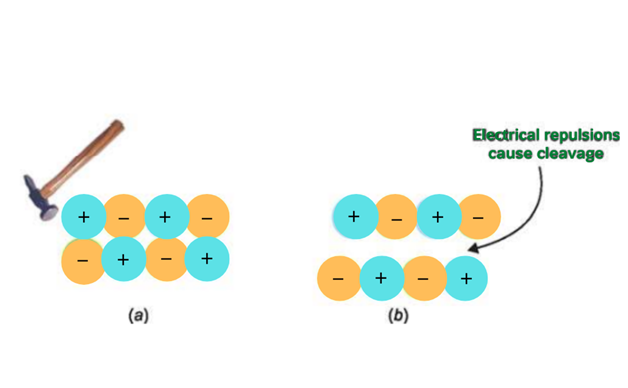
3. Hard and brittle
The crystals of ionic compounds are hard and brittle due to the strong force of attraction.
The crystals are made up of layers of cations and anions. These layers are in parallel arrangement thus ions of opposite charges lie in front of each other. When an external force is applied, ions of the same charges come across each other. Thus they start repelling each other and fall apart. The crystal cleaves at that position.
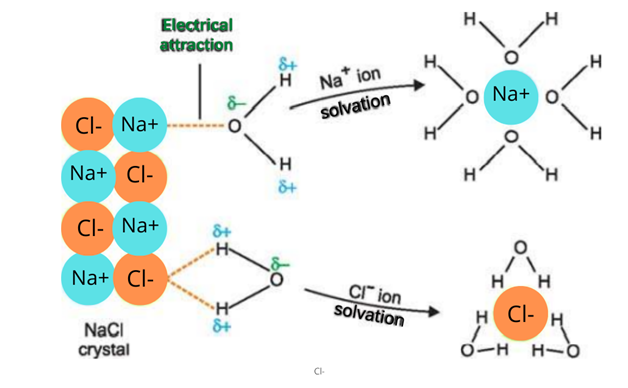
4. Solubility in water
When an ionic compound is placed in water, polar water molecules detach the positive and negative ions. These ions get surrounded by the water molecules and are thus soluble in water.
5. Conductivity
Solid ionic compounds are poor conductors of electricity. This is due to the reason that ions are fixed at their positions. In a molten state or water, ions get free from their position thus they conduct electricity. Thus molten ionic compounds or their aqueous solutions conduct electricity when placed in the electrolytic cell. Na+
6. Isomerism
The ionic bond is non-rigid and non-directional. Due to this reason, ionic compounds do not exhibit stereoisomerism.
7. Ionic reactions
Ionic compounds give reactions between the ions and these reactions are very fast.