Learning objectives
In this article, the author has explained alkali metals, the physical and chemical properties of alkali metals, and what is the trend of alkali metals on the periodic table.
What are alkali metals?
Table of Contents
The elements of group I-A except hydrogen whose oxides and hydroxides are strong alkalies are called alkali metals. These are Li, Na, K, Rb, Cs and Fr.
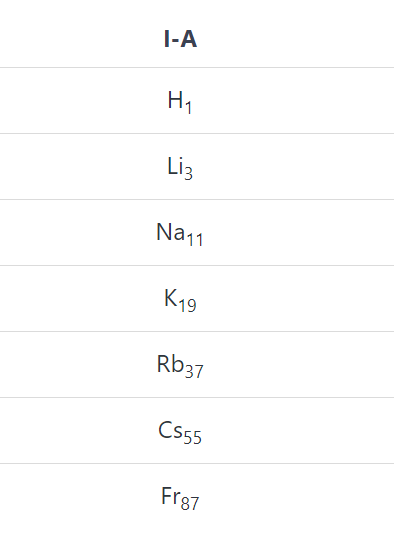
These are known as s-block elements as their outermost orbitals are s and only one electron is present in it. These are highly reactive metals that Na metal ignites as it comes in contact with atmospheric oxygen.
History of alkali metals
The name alkali came from the Arabic root which means ashes. The reason is that these metals particularly Na and K are present in the ashes of the planet. These produce a strong alkaline solutions in water.
Occurrence of alkali metals
Alkali metals don’t occur free in nature. This is due to the reason that are highly reactive.
Alkali metals and earth crust
Most of the earth’s crust is composed of insoluble aluminum silicates alkali metals.
Important minerals of alkali metals
Following are the names and formulas of some common minerals of alkali metals.
| Element | Mineral name | Chemical formula |
| Lithium | Spodumene | LiAl(SiO3)2 Contains 3.8-5.6 % of lithium |
| Sodium | Rock salt or Halite Chile saltpeter Natron Trona Borax | NaCl NaNO3 Na2CO3.H2O Na2CO3.2NaHCO3.2H2O Na2B4O7.10H2O |
| Potassium | Carnallite Sylvite Alunite (alum stone) | KCl.MgCl2.6H2O KCl K2SO4.Al2(SO4)3.4Al(OH)3 |
Deposits of lithium
The deposits of lithium are found in the form of complex minerals. These minerals are widely scattered over the earth. Spodumene whose formula LiAl(SiO3)2 is an important commercial source of lithium.
Occurrence of sodium and Potassium
Na and Potassium are the most abundant among the alkali metals. Each of these constitutes about 2.4% of earth’s crust.
Occurrence of rubidium and cesium
Small amounts of rubidium and cesium are found in the deposits of potassium.
Occurrence of francium
Francium has not been found in nature. It has been prepared artificially in the laboratory. It is very unstable. Very little is known about this metal.
Electronic configuration of alkali metals
Keeping in view the rules for the electronic distribution, all of these alkali metals have one electron in their outermost s-orbital.
Remember by s-block elements mean their outermost electron will enter the s-orbital.
By 1-A group means the alkali metals have one electron in their outermost s-orbital.
Following is the electronic configuration of alkali metals.

From the above image, it is clear that the alkali metals have only one electron in the outermost s-orbital, hence showing the valency of one. These can lose one electron easily and attain the electronic configuration of inert gases.
General properties of alkali metals
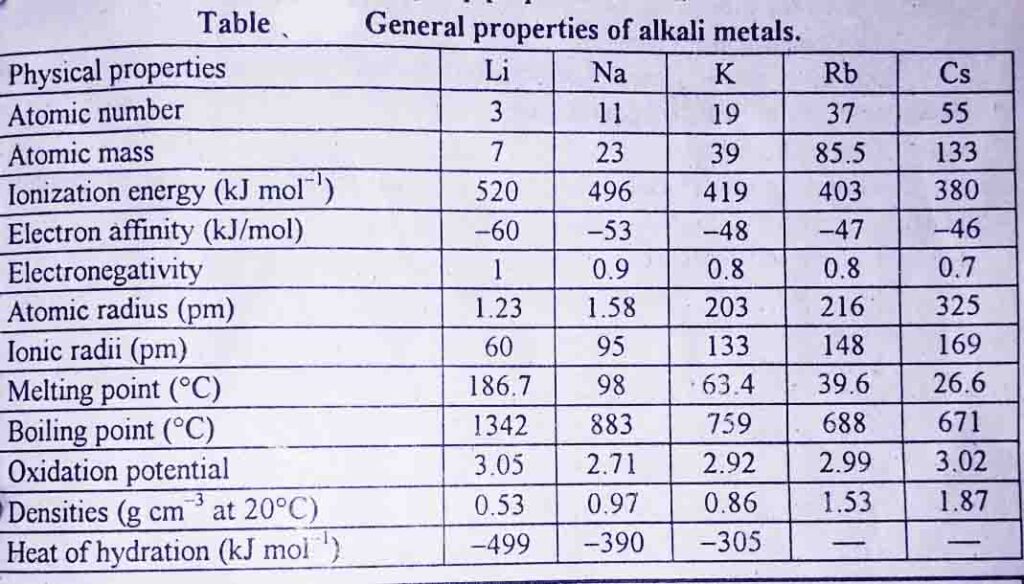
Physical properties of alkali metals and their trends in the periodic table
Alkali metals show similarity and gradual variation in physical properties in the periodic table as follows.
1: Physical state
Alkali metals are extremely soft. These can be easily cut with a knife and can be fused.
2: Metallic luster
When alkali metals are freshly cut, they have a bright luster. This luster is quickly tarnished as soon as metal comes in contact with the atmosphere.
3: Atomic volume
The atomic volumes of the alkali metals increase down the group. Their atomic radii and ionic radii also increase down the group systematically.
4: Ionization energies
Alkali metals have low ionization energies and values decrease down the group with an increase in size.
5: Electron affinity
Alkali metals have low electron affinities and the values decrease down the group because of an increase in atomic size.
6: Electronegitivity
Alkali metals are the least electronegative, and this decrease down the group because of an increase in atomic size.
7: Density
The alkali metals have low densities as compared to other metals of the periodic table. Li, Na, and K have densities less than that of water. The value of densities increases down the group from Li to Cs.
8: Melting and boiling points
Alkali metals have very low melting and boiling points. These low values are due to weak interatomic bonds in the solid-state. The melting and boiling points decrease down the group.
9: Electrical conductivity
Alkali metals have free electrons in the crystal lattice. These free electrons are responsible for electrical conductivity.
10: Oxidation state
The alkali metals lose one electron from the outermost orbital and show +1 oxidation states.
11: Colour of the flame
Whenever the alkali metals are heated on the flame, these give characteristic colour to the flame.
For example
- Lithium shows crimson-red color
- Sodium shows golden yellow color
- Potassium shoe violet color
- Rubidium and cesium show violet color
This property of alkali metals is called the flame test.
12: Color of the compounds
All the compounds of alkali metals are colorless except those compounds in which the negatively charged part of the molecule is colored.
For example KMnO4 and K2Cr2O7 are the colored compounds. Their colors are due to MnO4-1 and Cr2O7-2 .
13: Hydration of ions
The salts of alkali metals are hydrated. This is due to their sizes. The hydration is due to the high charge density of alkali metal ions. The charge densities of alkali metal ions decrease down the group, so the hydration goes on decreasing.
14: Reducing properties
Alkali metals are good losers of electrons, so these are good reducing agents. Whenever these react with water or acid these liberate H2. The oxidation number of hydrogen changes from +1 to zero.
The reducing property goes on increasing down the group. It means that cesium is the best reducing agent.
Chemical properties of alkali metals and their trends in periodic table
Let us discuss the chemical properties of alkali metals. These are highly reactive elements.
1: Reactivity
Alkali metals are highly reactive due to low ionization energies.
2: Formation of oxides
Alkali metals react with oxygen and the surface is tarnished. This is due to formation of oxides on the surface.
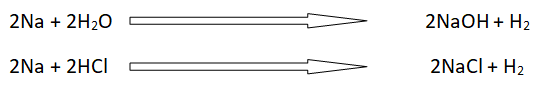
3: Reaction with water
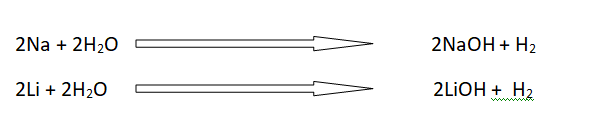
The reaction of alkali metals with water is very fast.
A small piece of Li, Na and K floats on water. These react vigorously and liberate hydrogen. They produce metal hydroxides. These reactions are highly endothermic. The energy which os produced can burn the hydrogen gas.

The reactivity of alkali metals with water becomes more and more vigorous as we move down the group.
Reactions of K, Rb, and Cs with water
These metals are so reactive with water that these react with ice at -100oC.
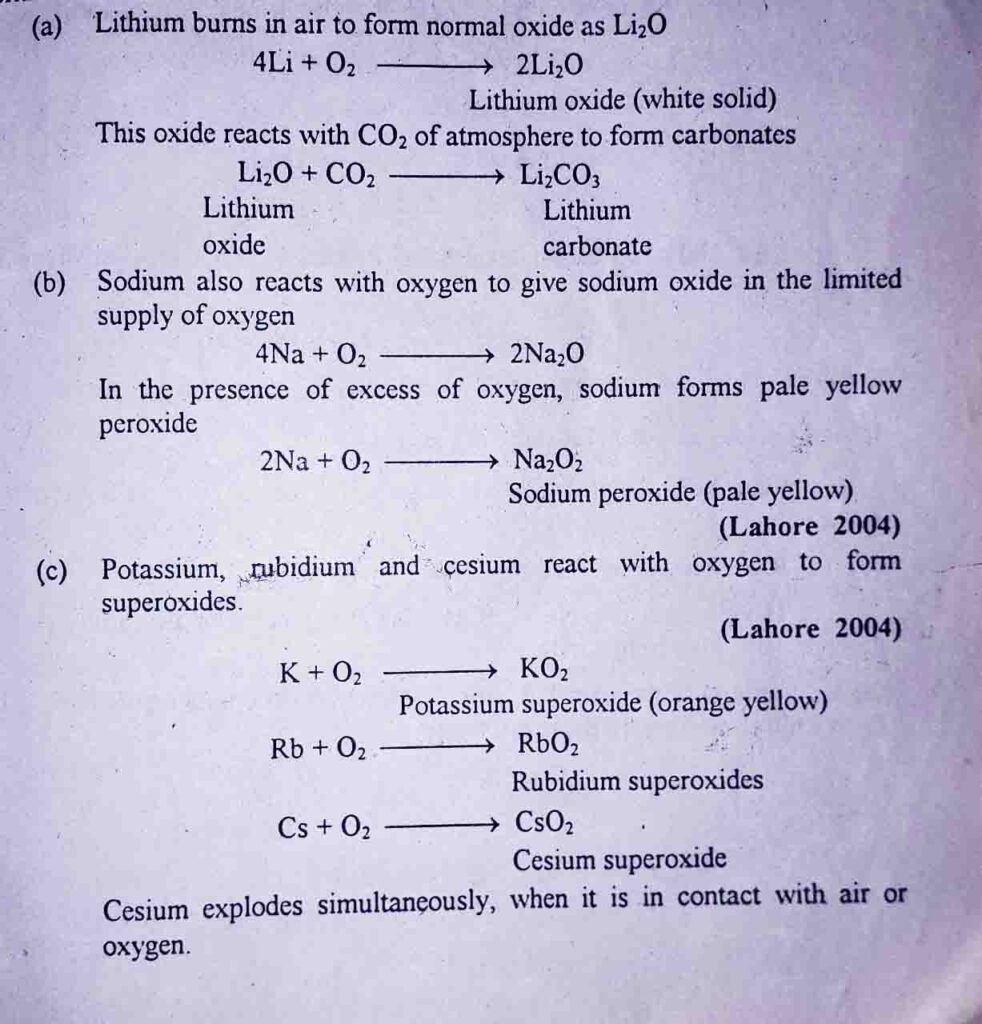
4: Formation of ionic hydrides
Alkali metals react with hydrogen to form ionic hydrides.
Lithium, sodium and potassium react with hydrogen nat higher temperature to form hydrides. Rb, and Cs react with hydrogen violently at room temperature.
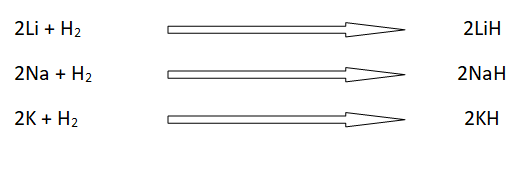
Lithium Hydride LiH, sodium hydiride NaH are useful source of hydrogen, when these react with water.
Ionic hydrides of group I-A elements give H- ions. H- ions is a good reducing agent.
5: Reaction with electronegative elements
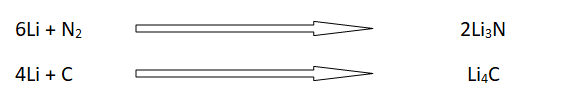
The elements like N2, C, S, and halogens react with I-A group elements to give respective compounds.
For example, lithium is the only I-A group element that combines with N2 and carbon to form nitride and a carbide respectively.
Reaction with halogens
Alkali metals react with halogens to give halides. Li and Na react slowly with chlorine at room temperature to give LiCl and NaCl. Molten Na burns with a brilliant yellow flame in the atmosphere of chlorine to form NaCl.

K, Rb and Cs react vigorously with all the halogens to form metal halides.
Reaction with sulfur
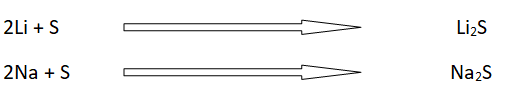
All the alkali metals react with molten sulfur to form metal supfides.
For example