A versatile and simple method for the synthesis of chiral primary, secondary, and tertiary amines containing two stereogenic centers has been developed by the Biocatalysis group at the University of Amsterdam.
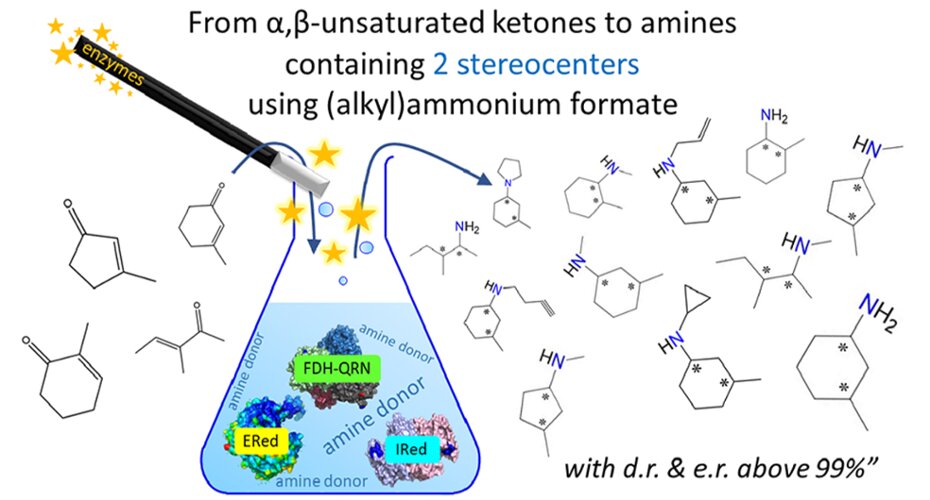
The enzymatic process using a biocatalytic system consisting of ene-reductases (EReds) and reductive aminases (IReds/RedAm) is a very interesting and effective approach to the preparation of optically active amines.
A recent study by researchers Tanja Knaus, Francesco Mutti, and Maria Luisa Corrado shows that your work has been recognized. It appears on the cover of the journal’s print edition.
The research that’s been done so far in the area of biocatalytic conversion represents a significant step forward, not only for the group’s portfolio but for the field in general.
Pharmaceuticals often contain amines with two or more stereogenic centers, and the development of stereospecific reactions for their synthesis is one of the most important advances in the field.
Biocatalytic methods for the synthesis of amino acids are superior in terms of enantiospecificity (stereoselectivity) and require much less harsh reaction conditions as compared to chemical methods.
In general, the developed cascade strategy allows sustainable chemical synthesis by reducing the amount of reagents used and the quantity of waste.
The only reagent used is ammonium or alkylammonium formate, serving as amino donor, as the source of reducing equivalents.
This was enabled by incorporating a novel NADP-dependent FDH into the cascade reaction.
As scientists continue their quest for more effective and stable catalysts, they should keep in mind that the next breakthrough is only a click away.
An innovative effort to reduce the use of fossil fuels to produce renewable chemicals.