What is sulfonation?
Table of Contents
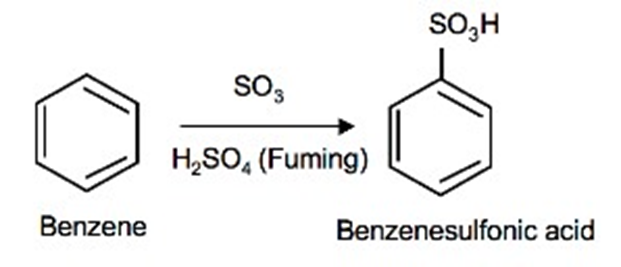
The addition of sulfur trioxide (SO3) into benzene is called sulfonation of benzene.
Equation
Explanation
Sulfonation of benzene is an example of electrophilic aromatic substitution reactions just like nitration. Sulfonation of benzene takes place when benzene reacts with sulfur trioxide (SO3). In this reaction sulfuric acid acts as a catalyst.
Catalyst is specie that increases the reactivity of reagents which takes part in the chemical reaction.
Mechanism of sulfonation of benzene
The mechanism of sulfonaton of benzene completes in three steps which are given below
STEP 1: Formation of electrophile (SO3)
When two moles of sulfuric acid reacts the electrophile (SO3) is formed along with the formation of hydronium ion and sulfonate ion (HSO–4).
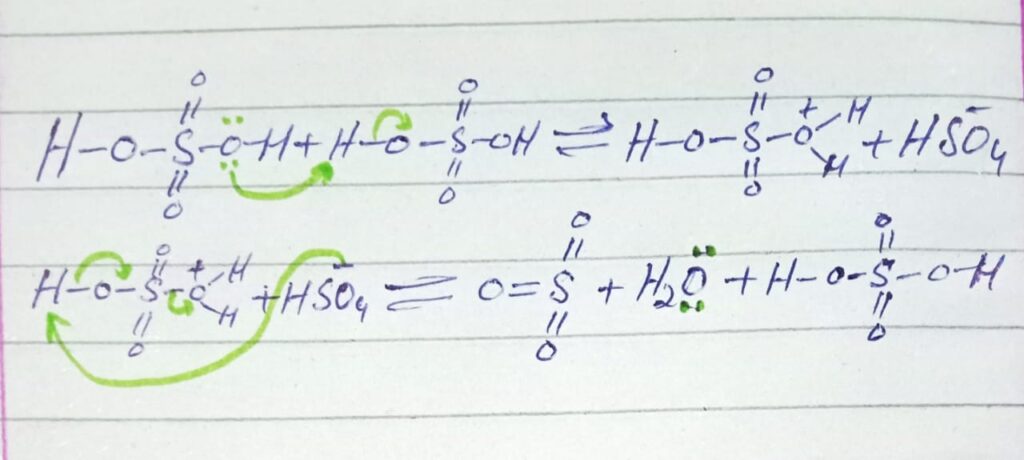
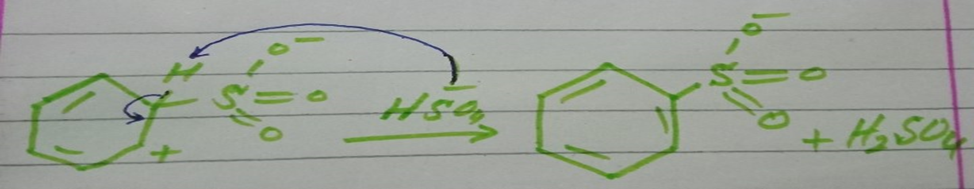
Step 2 Attack of electrophile
In this step electrophile attacks on the benzene ring to give sigma complex.

Step 3 Loss of proton
In this step sulfonate ion accepts proton from sigma complex to form aromatic ring.
Step 4 protonation of sulfonic acid group
In the last step sulfonic acid group accepts proton from hydronium ion (H3O+).
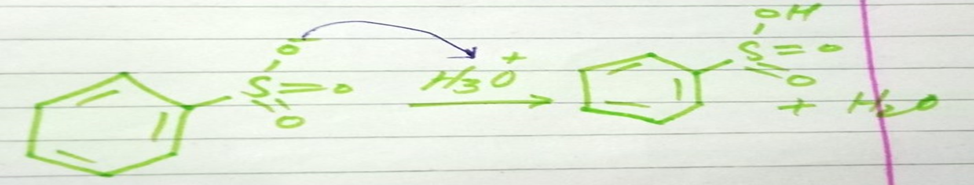
The final product is benzene sulfuric acid is formed.
FAQS
Why SO3 is used in sulfonation?
Conventionally sulfonation is done by sulfuric acid or oleum. But with SO3 sulfonation process has the following advantages. It is more direct and considerably faster than the present process. It requires fewer man hours and, therefore, is more economical.
Is sulfonation of benzene addition reaction?
Sulfonation is a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. The reaction is reversed by adding hot aqueous acid to benzenesulfonic acid to produce benzene.
Which reagents are necessary for the sulfonation of benzene?
There are two equivalent ways of sulfonating benzene: Heat benzene under reflux with concentrated sulfuric acid for several hours. Warm benzene under reflux at 40°C with fuming sulfuric acid for 20 to 30 minutes.