LEARNING OBJECTIVES
In this article, the author has explained what is a limiting reactant, definition, examples and method to find a limiting reactant in during a chemical reaction.
Limiting reactant Definition
Table of Contents
In a chemical reaction, the limiting reactant is the reactant that is completely consumed when the reaction is complete.
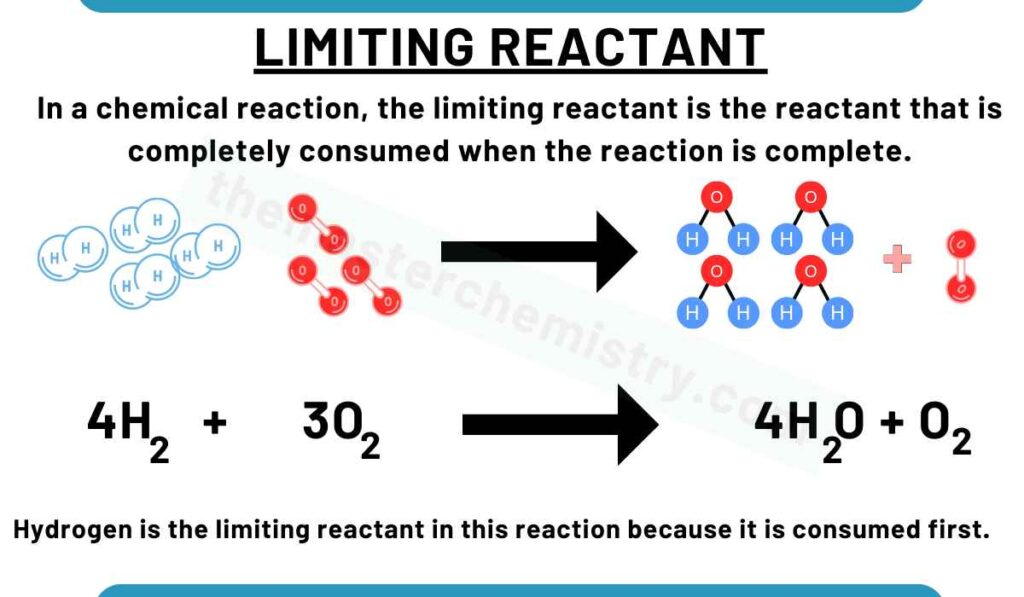
Watch the video lecture to better understand about limiting reactant
Explanation of limiting reactant
If stoichiometry amounts are put in chemical reactions, these amounts are completely used. Sometimes, stoichiometric amounts required by the reaction are not used during the reaction for the following purposes:
- To ensure that whole of the expensive reactant is completely consumed
- To make the reaction occur faster
- To complete 100% reaction
- To measure the yield of a balance chemical reaction
When we use non-stoichiometric amounts, one of the reactants is consumed earlier than the other. The reactant which is used in less amount is of products formed and is called a limiting reactant.
Related: Avogadro’s number and Molar Volume-Themasterchemistry.com
How to find limiting reactant?
There are three main ways to determine the limiting reagent in a chemical reaction:
1. By looking at the number of moles of each reactant
Here is how find limiting reactant with moles:
- Determine the balanced chemical equation for the chemical reaction.
- Convert all given information into moles (most likely, through the use of molar mass as a conversion factor).
- Calculate the mole ratio between the reactants.
- The reactant with the smaller number of moles is the limiting reagent.
2. By calculating and comparing the amount of product each reactant will produce
- Determine the balanced chemical equation for the chemical reaction.
- Convert the given information into moles.
- Use stoichiometry to calculate the mass of product produced by each reactant.
- The reactant that produces the lesser amount of product is the limiting reagent.
3. By using the excess reactant method
- Determine the balanced chemical equation for the chemical reaction.
- Convert the given information into moles.
- Calculate the amount of excess reactant that is left over after the reaction is complete.
- The reactant that does not have any excess is the limiting reagent.
Examples of limiting reactant
Example 1
In a chemical reaction, a large quantity of oxygen makes things burn rapidly. Since oxygen used in excess is left behind when the reaction is completed.
The other reagent is consumed completely. That reactant that is consumed earlier is known as limiting reactant.
Example 2
Imagine you have a recipe for a cake that requires 1 cup of flour and 2 cups of sugar. If you only have 1 cup of flour, then the flour is the limiting reactant.
This means that you can only make a cake with 1 cup of flour, even though you have enough sugar. The sugar will be left over.
Example 3
Imagine you have 30 eggs and 58 slices of bread, and you want to make sandwiches.
You know that you need 1 egg and 2 slices of bread to make 1 sandwich. This means that you can make a maximum of 29 sandwiches, because you don’t have enough bread slices to make 30 sandwiches.
The 58 slices of bread will be completely used up, while you will be left with 1 egg. Therefore, the bread slices are the limiting reactant.
Example 4
Consider the reaction along with stoichiometric amounts:
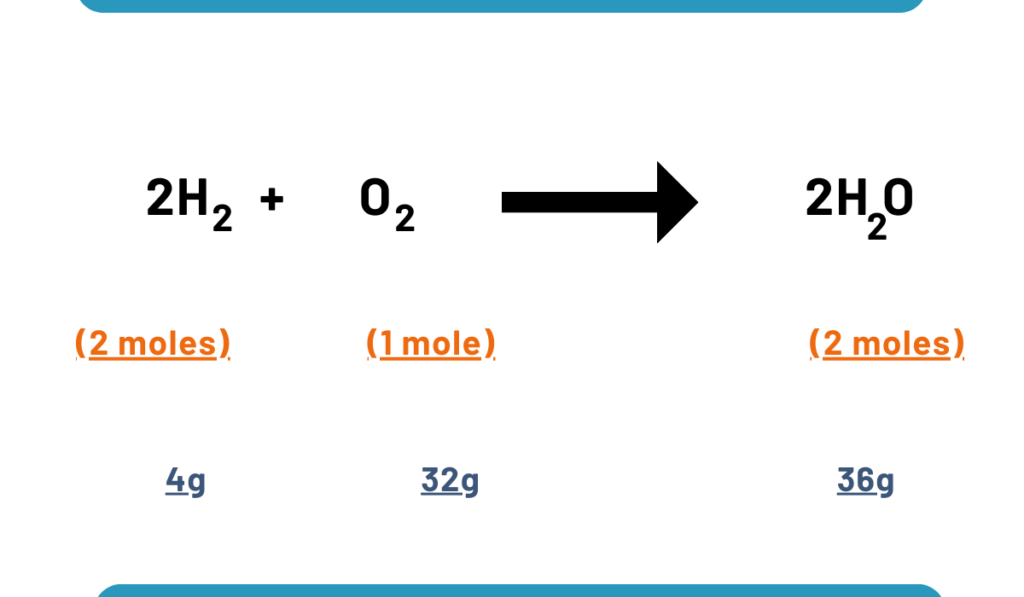
If we use stoichiometric amounts then 4g amounts of hydrogen and 32 g of oxygen, we get 36g of water.
But if we take non-stoichiometric amounts say 4g of H2 and 60g of oxygen, we will not get more quantity of water than 36g because H2 is less. So H2 is a limiting reactant. 28 g of oxygen will be left unreacted.
2H2 + O2 ——-> 2H2O
4g 36 g 36g
Why concept of limiting reactant is not applicable to the reversible reactions?
The concept of limiting reactant is not applicable to reversible reactions because in a reversible reaction, the reactants and products can interconvert.
This means that the amount of each reactant and product present at equilibrium will depend on the initial concentrations of the reactants, the temperature, and the pressure.
In a non-reversible reaction, the limiting reactant is the reactant that is completely consumed when the reaction reaches equilibrium. This is because the reaction can only proceed in one direction, and the limiting reactant determines how much of the other reactants can be consumed.
In a reversible reaction, however, the reaction can proceed in both directions. This means that the limiting reactant will not be completely consumed, and there will be some of each reactant and product present at equilibrium.
For example, consider the reversible reaction of hydrogen and iodine to form hydrogen iodide:
H2 + I2 <=> 2HI
If we start with equal amounts of hydrogen and iodine, the reaction will proceed until the concentrations of hydrogen and iodine reach equilibrium.
At equilibrium, there will be less hydrogen and iodine than there were initially, but there will still be some of each present.
In this case, there is no limiting reactant. The amount of hydrogen iodide produced will depend on the initial concentrations of hydrogen and iodine, the temperature, and the pressure.
Limiting reactant problems
Problem 1
NH3 gas can be prepared by heating together two solids, NH4Cl and Ca(OH)2. If a mixture containing 100 g of each solid is heated, then
a) Calculate the number of grams of NH3 produced
b) Calculate the excess amount of reagent left unreacted
Solution
The balanced chemical equation for the chemical reaction is
2NH4Cl + Ca(OH)2 —-> CaCl2 + 2NH3 + 2H2O
a) Number of grams of NH3 produced
given mass of NH4Cl = 100g
Molar mass of NH4Cl = 14+1×4+35.5 = 53.5 g/mol
Number of moles of NH4Cl= 100/53.5 = 1.87 moles
Given mass of Ca(OH)2 = 100 g
Molar mass of Ca(OH)2 = 40 + 2(16+1) = 74 g
Number of moles of Ca(OH)2 = 100/74 = 1.35 moles
–> Compare NH4Cl and NH3 according to a balanced chemical equation.
NH4Cl : NH3
2 moles : 2 moles
Therefore 1.87 moles: 2/2 x 1.87 = 1.87 moles
–> Compare Ca(OH)2 and NH3 according to the balanced chemical equation
Ca(OH)2 : NH3
2 moles : 2 moles
Therefore, 1.35 moles: 2 x 1.35= 2.70 moles
Since NH4Cl produces the least amount of NH3, hence NH4Cl is the limiting reactant.
Thus no. of moles of NH3 produced= 1.87 moles
Molar mass of NH3 = 14+1 x3 = 17 g/mol
Hence the amount of NH3 produced = No.of moles x Molar mass of NH3 as given below.
Amount of NH3 produced = 1.87 x 17 = 31.79 g
b) Amount of reagent left unreacted
–> Compare NH4Cl and Ca(OH)2 according to a balanced chemical equation.
NH4Cl : Ca(OH)2
2 moles : 1 mole
1.87 moles : 1×1.87/2 = 0.935 moles
Moles of Ca(OH)2 taken = 1.35 moles
Therefore unreacted moles = 1.35 – 0.935 = 0.415 moles
Thus mass of Ca(OH)2 left = 0.415 x 74 = 30.71 g
Problem 2
Calculate the number of grams of Al2S3, which can be prepared by the reaction of 20 g of Al and 20g of sulfur. How much non-limiting reactant is in excess?
Solution
The balanced chemical equation for the chemical reaction is
2Al + 3S —–> Al2S3
Given mass of Al = 20 g
Number of moles of Al = 20/27 = 0.74 moles
Given mass of S = 30 g
Number of moles of S = 30/32 = 0.9375 moles
Determination of limiting reactant in this reaction
Compare Al and Al2S3 according to a balanced chemical equation.
Al : Al2S3
2 moles : 1 mole
Therefore, 0.74 moles: ½ x 0.74 = 0.37 moles
Compare the number of moles of the product produced by Al = 0.37
–> Compare S and Al2S3 according to a balanced chemical equation.
S : Al2S3
3 moles : 1 mole
Therefore, 0.9375 moles: 1/3 x 0.9375 = 0.3215 moles
Hence the number of moles of Al2S3 produced by S = 0.3225 moles
Since S produces the least number of moles of the product, therefore it is the limiting reactant.
Hence moles of Al2S3 produced = 0.3125 moles
Mass of Al2S3 produced = 0.3125 x 150 = 46.87 g
Determination of amount of Al left unreacted
2Al + 3S ——> Al2S3
–> Compare the moles of S and Al to find the moles of Al reacted
S : Al
3 moles : 2 moles
0.9375 moles: 2/3 x 0.9375 = 0.625 moles
Moles of Al consumed = 0.625 moles
Moles of Al taken = 0.74 moles
Moles of Al left unreacted = 0.74 – 0.625 = 0.115 moles
Mass of Al left unreacted: 0.115 x 27 = 3.105 g
Related questions
How does a limiting reactant help to control the reaction?
The limiting reactant helps to control the reaction by determining how much of the other reactants can be consumed. In a chemical reaction, the reactants are converted into products according to a set of specific proportions. The limiting reactant is the reactant that is present in the smallest amount relative to the other reactants. This means that the limiting reactant will be used up first, and the other reactants will be left over in excess.
11g of carbon is reacted with 32 g of oxygen to give CO2. Which is the limiting reactant?
C + O2 ——> CO2
According to a balanced chemical equation, there should be 44 grams of CO2 after the complete reaction of 12g of carbon with 32 grams of oxygen. Therefore, 32 grams of oxygen will be in excess compared to 11 grams of carbon. So in this case carbon is a limiting reactant.
Why the concept of limiting reactants is not applicable to reversible reactions?
The concept of limiting reactants is not applicable to reversible reactions because the reactants and products are interconverted at equilibrium.
How do you find the limiting reactant?
You need to perform a titration analysis to determine the limiting reactant. For example, if you have two reactants, A and B, then you can determine the limiting reactant by using a stoichiometric equation, such as (A + B) = C.
There are three main ways to find the limiting reactant in a chemical reaction:
- By looking at the number of moles of each reactant.
- By calculating and comparing the amount of product each reactant will produce.
- By using the excess reactant method.
What is a limiting and excess reactant?
In a chemical reaction, the limiting reactant is the reactant that is completely consumed when the reaction is complete. The excess reactant is the reactant that is left over after the reaction is complete.
The limiting reactant determines the amount of product that can be formed. If you start with less than the stoichiometric amount of a reactant, then that reactant will be the limiting reactant and the reaction will stop when it is completely consumed. The other reactants will be left over in excess.
The excess reactant does not affect the amount of product that can be formed. It simply means that there was more of that reactant than was needed to react with the limiting reactant.
what is the formula of the limiting reagent
There is no formula for the limiting reagent. The limiting reagent is the reactant that is completely consumed when the reaction is complete. It is determined by calculating the number of moles of each reactant and comparing it to the stoichiometric coefficients in the balanced chemical equation.
The reactant with the smaller number of moles is the limiting reagent.