LEARNING OBJECTIVES
Table of Contents
In this article, the author has explained measurement of vapor pressure throuh manometric method.
Imagine that you are in a chemistry lab. You have been tasked with measuring the vapor pressure of an unknown liquid, but you do not know how to go about it. How will you measure the vapor pressure? If this is your first time being faced with this measurement task, then don’t worry! This blog post will help teach you how to measure vapor pressure so that when it comes up again in class or during an exam, you’ll be prepared.
The measurement of vapor pressure is important in many fields, including chemical engineering and meteorology. It’s also one of the most difficult to measure accurately.
Watch the video to better understand the measurement of vapor pressure
Methods to measure the vapor pressure
Four methods exist to measure vapor pressure: the dynamic method, the equilibrium or static method, and a modification of the dynamic and manometric method. The manometric method is the most widely used method to measure vapor pressure.
Dynamic Method
This method is the most common and requires a balance or pressure transducer. The sample container has two ports, one for pressure measurement and the other to allow entry of air[1].
Equilibrium Method
The equilibrium method uses a closed system in which all gas present initially (before heating) will be removed when the temperature reaches that of boiling point.
Modified Dynamic Method
This method is also called the falling-ball viscometer method since it measures pressure as a marble suspended by a thread falls through the liquid of known viscosity and vapors present in the atmosphere above them. A digital camera records the time taken for the marble to reach the bottom which gives an idea about vapor pressure.
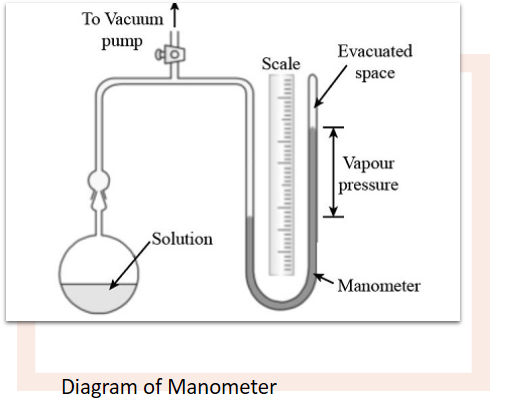
Measurement of vapor pressure through Manometric method
The manometer is a type of instrument that measures the difference in pressure between two points. The upper reference point is called static pressure and the lower one is known as dynamic pressure.
For example, if we consider a weather barometer then the upper end of the tube is open to the atmosphere and the lower end is connected to a container having mercury.
When atmospheric pressure increases, more molecules will collide with it resulting in a higher level of liquid inside the glass tube. So, the pressure of the atmosphere is acting at its upper end and thus it’s called static pressure.
Working of Manometric method
The liquid whose vapor pressure is to be measured is kept in a flask which is placed in a thermostat to control temperature. One end of the tube coming from the flask is connected to a manometer. the other end is connected to a vacuum pump. the liquid is frozen using a freezing mixture. the space above the frozen mixture is evacuated.
In this way, air and vapors of liquid are removed from the surface of the liquid. The frozen liquid is melted by a thermostat to release the trapped air. Air is again removed in the same way. The liquid is melted again.
The same process is repeated multiple times to remove the air from the flask.
When you heat liquid again, it gets to the temperature at which the vapor pressure of the liquid is measured. It takes up space in one part of the manometer. The other part that faces atmospheric pressure goes up and takes more space than before.
Mathematically it can be represented as
P= Pa + Δh
In the above equation, p is the vapor pressure of the liquid at 1 atmospheric pressure
Pa is atmospheric pressure
Δh is a difference in the heights of the Hg column in two minutes.