Written by Adeel Abbas
Definition
Table of Contents
Those groups that direct incoming groups to ortho and para positions are called ortho-para directing groups.
In other words “Electron donating groups in the aromatic ring are called ortho-para directing groups.”
For better understand also read my article on meta directing groups.
Examples of ortho-para directing groups
Some of the important ortho-para directing groups are –OH, -NH2, -OCH3, -Cl, -Br, -I.
Actually, these groups facilitate the availability of electrons to the electrophiles in the ortho and para positions by releasing electrons to the benzene ring.
Because these are electron-donating groups they donate the electrons to the benzene ring. Benzene becomes electron rich at the ortho and para positions. Therefore incoming electrophile attacks at the ortho and para positions.
As a result, the benzene ring is more chemically reactive toward electrophiles.
So the benzene ring can provide new incoming groups with many locations.
How electron donating groups direct electrophile at ortho and meta position?
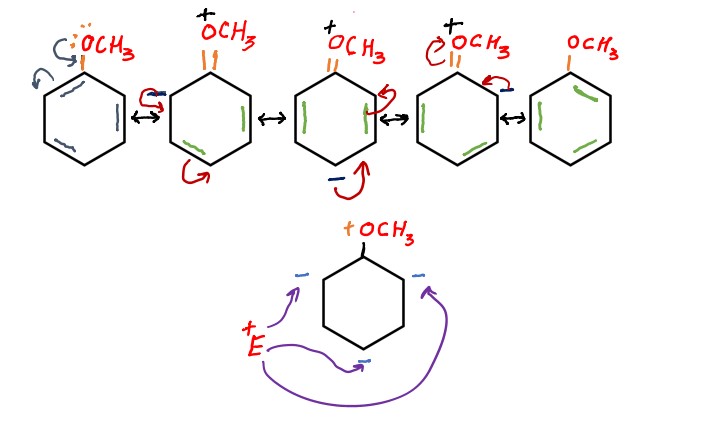
It is cleared from above mechanism how ortho-para directing groups directs the incoming electrophile at ortho and para position.
Examples of ortho-para directing groups
Nitration of toluene
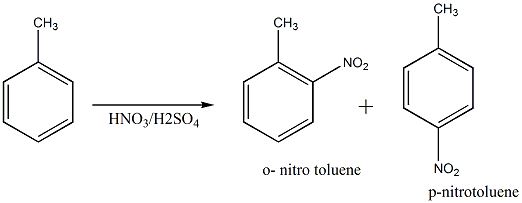
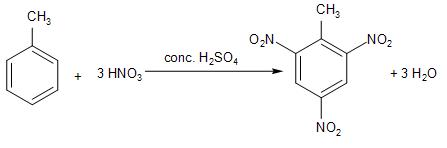
The electron-releasing effect of the methyl group is significant and it makes the ring a good nucleophile. Due to this increased reactivity more nitro groups can enter the ring.
Nitration of Anisole
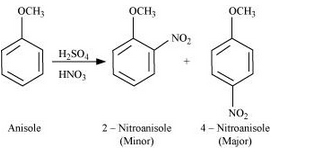
-OCH3 groups is electron donating group due to lone pair on oxygen atom. it gives us two products where electrophile nitro group is attached at ortho and para direction.