Written by Adeel Abbas
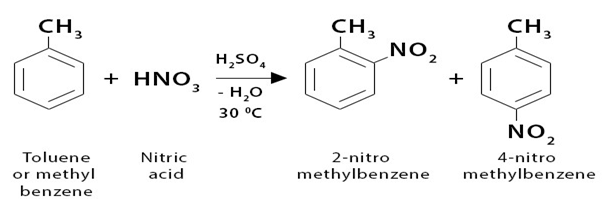
The introduction of a nitro group into toluene is called nitration of toluene. Nitro group acts as a electrophile.
Explanation:
Table of Contents
Toluene reacts around 25 times more quickly than benzene in the nitration reaction. In order to prevent the substitution of more than one nitro group, a lower temperature would be used in this instance—30°C as opposed to 50°C. The procedure, which uses the same concentrated nitric and sulphuric acid nitrating mixture, is otherwise identical.
Essentially, two isomers, 2-nitromethylbenzene and 4-nitromethylbenzene, are produced. The content of 3-nitromethylbenzene is just 5% or so of the product. The term “2,4-directing” refers to methyl groups. Which is also called ortho para director.
Mechanism:
Step 1: Formation of nitronium ion
Nitric acid and sulfuric acid react to produce the nitronium ion. Nitric acid will protonate in the presence of sulfuric acid, causing water molecules to evaporate and nitronium ions to form. The aromatic ring attacks the potent electrophile nitronium ion fast.
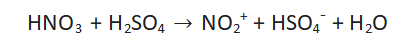
Step 2: Attack the electrophile to give the sigma complex
The benzene ring is attacked by the electrophile (nitronium ion) in this stage, resulting in the creation of the sigma complex.
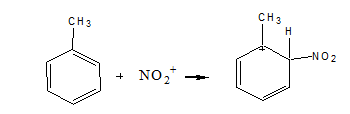
Step 3: Loss of proton to give nitrotoluene
The sulfonate ion takes a proton from the arenium ion in this stage, producing nitrotoluene.
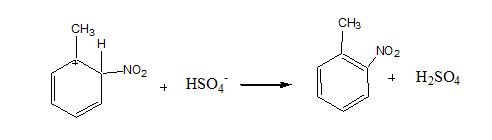
This is the final product which is called nitro toluene.
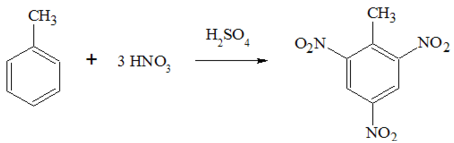
Formation of Trinitrotoluene
German chemist Julius Wilbrand created TNT for the first time in 1863, and it was initially employed as a yellow dye. It took three decades for its potential as an explosive to be realized, mostly because it was more difficult to detonate and less sensitive than alternatives.
TNT is manufactured in an industrial setting in three steps. To create mononitrotoluene, toluene is first nitrated with a solution of sulfuric and nitric acid (MNT). After being separated, the MNT is reiterated to create dinitrotoluene (DNT). The DNT is nitrated to trinitrotoluene (TNT) in the last phase using an anhydrous solution of nitric acid and oleum. The production method uses nitric acid, however, the diluted sulfuric acid can be concentrated again and utilized again. Following nitration, TNT is stabilized using a procedure known as sulfation, in which the crude TNT is treated with an aqueous sodium, sulfite solution to get rid of any less stable isomers of TNT and other undesirable reactions by-products.
FAQs
What type of reaction is nitration of toluene?
Before answering this question, we should know that nitration of Toluene involves an electrophilic aromatic substitution reaction. It involves the addition of nitro in the ortho and meta position of an aromatic compound.
Why the para product is major in the nitration of toluene?
In case of stability: Para product is more stable because is away from the substituent group so it does not affect by steric hindrance as the ortho position I’d more steric hindered.
At which position does the nitration of toluene take place?
Nitration takes place at the ortho and para position in Toluene to form Nitrotoluene isomers. When the formed isomer is heated it will give Dinitrotoluene and finally be converted into Trinitrotoluene which is very explosive.
What is the nitration temperature of toluene?
That means that you would use a lower temperature to prevent more than one nitro group from being substituted – in this case, 30° rather than 50°C. Apart from that, the reaction is just the same – using the same nitrating mixture of concentrated sulphuric and nitric acids.
Why toluene is ortho and para directing?
In toluene, a methyl group is activating and ortho-para directing because it increases electron density at ortho and para positions.
Why nitration of toluene is easier than benzene?
Solution: The methyl group in toluene is an activating group. It increases the electron density in ortho and para positions and hence, an electrophile readily attacks the position. The presence of an ortho para directing group increases the rate of reaction, Hence, nitration of toluene is faster than benzene.
What does TNT consist of?
Toluene is a clear, colorless liquid that evaporates when exposed to room-temperature air. Nitric acid (HNO3) is a highly corrosive mineral acid also known as strong water. Sulfuric acid, sometimes known as oleum, is an oily, colorless liquid.