In this article, the author has discussed the oxidation state. After reading this article you will learn
- What is the oxidation state?
- How to find an oxidation state?
- Oxidation state in ionic and covalent compounds
- Relationship of oxidation state and group number
- The oxidation state of transition elements
- Variable oxidation states
What is Oxidation state?
Table of Contents
Oxidation state of an atom in a compound is the charge which it would carry in the most probable ionic formulation of ionic compound.
It is also referred as the number of electrons an element takes or gains during a chemical reaction.
For example in case of ionic compounds such as NaCl, Na is a metal and Cl is gases. We know that metals always lose electrons and gases takes gain the electrons.
In this case, Na donates its one electron to chlorine and develops a +1 charge that is its balance as well.
Similarly, Cl gains one electron and develops a -1 charge that becomes its valency.
Oxidation number in covalent compounds
In covalent compounds the positive and negative oxidation number of an element is decided on the basis of electronegitivity of the elements. SnCl is a covalent compound. The oxidation number of Sn is +4 and that of Cl is -1.
The oxidation number of an atom is zero when it is present in the form of a free element.
How to find oxidation state?
Oxidation state of an atom can be predicted by the number of its valence shell electrons .
The electronic distribution of elements in a particular group remains the same. The number of electrons in a valance shell mostly decide about the oxidation state.
Oxidation state rules for Calculating oxidation state
First of all try to know which group an atom belongs to?
Secondly see that does the atom lose electrons (metal) or gains electrons (non-metal, gases).
Following examples will clear this concept..
i) Elements of group I-A which have one electron in outermost shell show +1 oxidation state. Now as these are metals and lose that one electron from s-orbital.
Therefore loss of electron results into positive charge and only one electron is removed therefore, +1 is the oxidation state.
For example NaCl, KCl, KBr, NaBr.
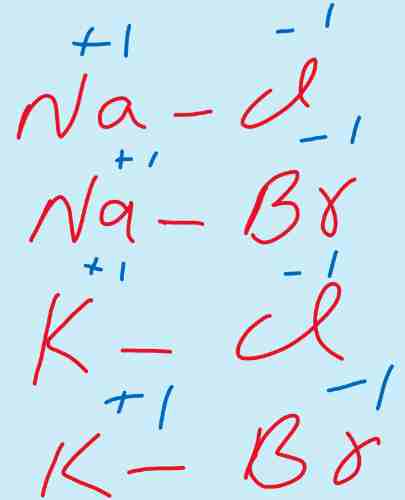
ii) The elements of the group II-A show the oxidation state +2 because they have only two valance electrons which they tend to lose during a chemical reaction.
For example, MgCl2, CaCl2, CaO,MgO etc.
Mg belongs to group II-A that means it has two electrons in valance shell. And it is metal that means it will lose the electrons.
Similarly Cl belongs to group VII-A that means it has 7 electrons in valance shell. And it is a non metal with higher electronegitivity that means it will gain the electrons. As in MgCl2 there are two Cl atoms, both the atoms will gain one electron each from Mg atom.
Same is the case with the Calcium chloride.
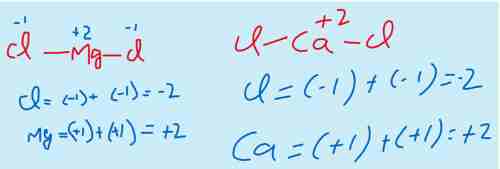
iii) The elements of the group III-A show the oxidation state of +3.
For example, AlCl3.
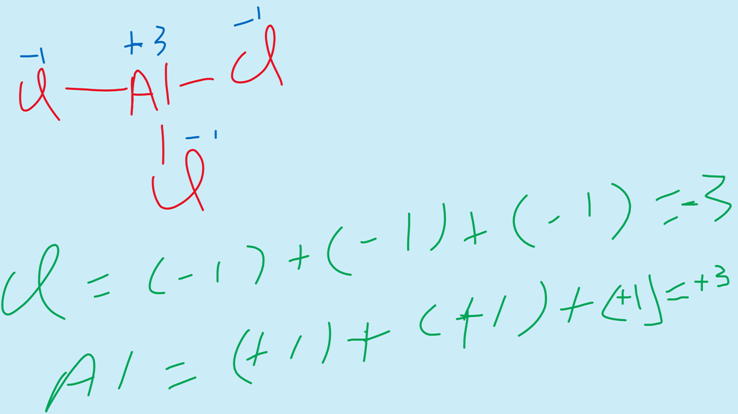
iv) The elements of group IV-A show the oxidation state of +4.
For example
CCl4
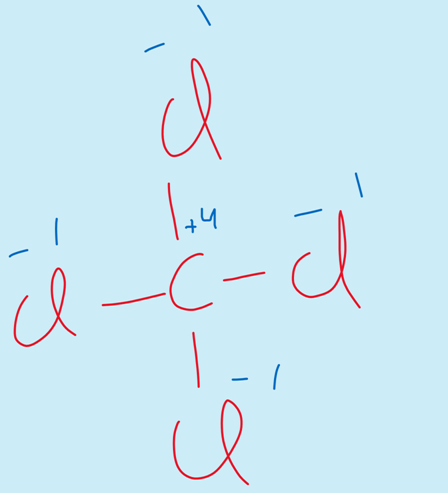
Some exceptions while finding oxidation state
Elements of group IV-A are not much electropositive that they always show the positive oxidation sate. When they combine with electronegative elements like Fluorine, chlorine, Bromine and Iodine etc, they show positive oxidation states.
But in metal carbides the oxidation state shown by Carbon is negative.
For example in case of calcium carbide CaC2.
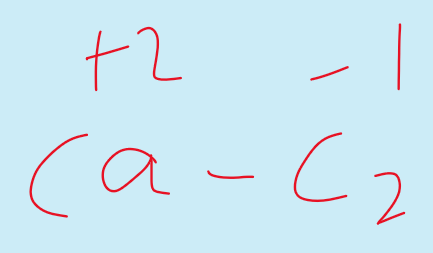
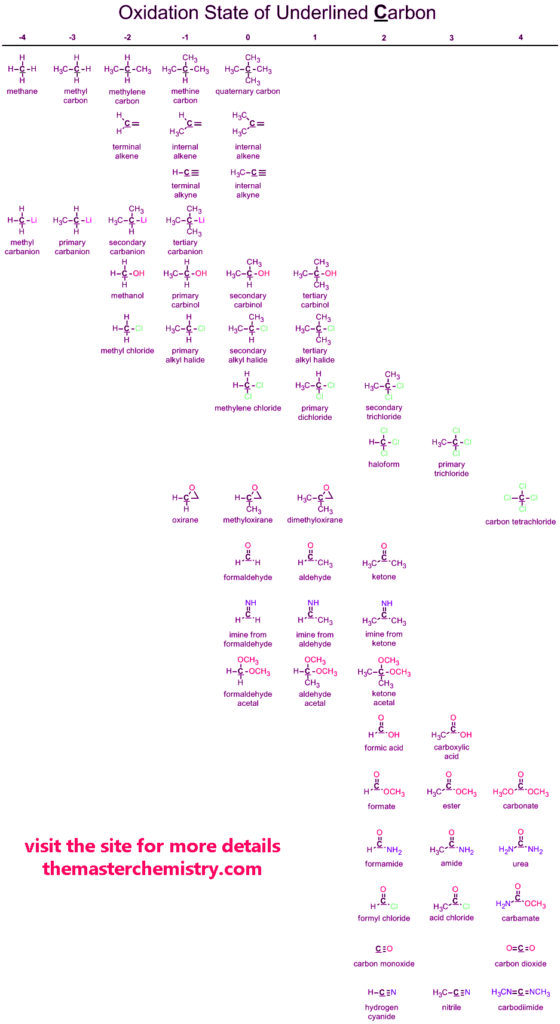
Oxidation states of Carbon
Carbon is the most important element of the periodic table. Carbon in many ways plays a very important role in the world. It has tendencies to show positive and negative oxidation states.
Below is given the complete data chart of carbon oxidation states.
iv) The elements of group V-A i.e., N, P, etc show two types of oxidation states that are +5 and -3.
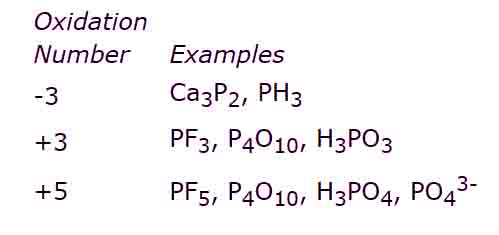
Vi) The elements of group VI-A i.e., O, S etc show the oxidation states -2, +4, +6.
For example in case of H2S, the oxidation state of Sulfur is -2. In SO2 it is +4 and in SO3 and H2SO4 the oxidation number of S is +6.
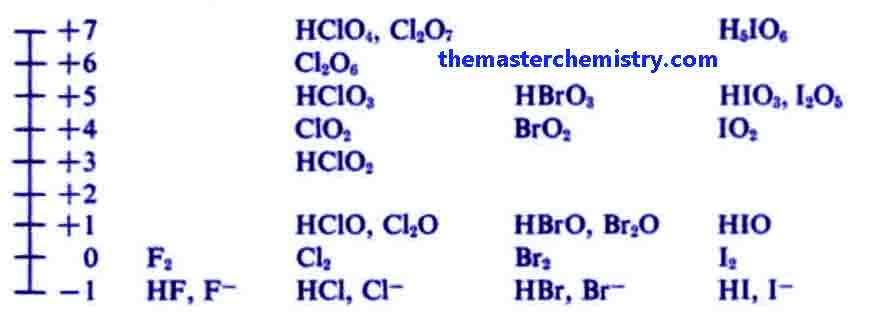
Vii) The elements of group VII-A show -1, +3, +5, +7 oxidation numbers.
Normally the elements of group 7-A show -1 oxidation state such as in case of NaCl, KCl, KBr etc.
But these elements also show varied oxidation state such as +3, +5, +7.
For example in the below diagram the elements of group 7 show varied oxidation state in different compounds.
Viii) The elements of group VIII-A or zero group show zero oxidation state. The reason is that the outermost shells are completely filled and have least tendencies to gain or lose the electron.
Oxidation state of transition elements
Transition elements are present in B sub-groups of the modern periodic table. They also show the oxidation state according to their group number. For example:
i) Cu, Ag, Au are present in group I-B, so their Oxidation states of Transition elements states are +1 and +3.
For example in case of CuCl2, CuO, CuSO4 Copper has +2 oxidation state which is most common.
In case of CuCl, Cu₂O, and Cu₂S copper has +1 oxidation state.
In case of KCuO₂ and K₃CuF₆ copper has +3 oxidation state.
In case of Cs₂CuF₆ copper has +4 oxidation state.
ii) Zn, Cd, Hg are present in II-B, so their oxidation states are +2.
For example in case ZnCl2, CdCl2 zinc and cadmium has +2 oxidation state.
iii) vanadium is present in group V-B and shows the oxidation number of +5.
For example in case of V2O5 Vanadium shows +5 oxidation state.
iv) Cr is present in VI-B and has +6 oxidation state.
For example in case of CrO3, H2CrO4, and H2Cr2O7 Chromium has +6 oxidation state.
V) Mn is present in VII-B group and hass +7 oxidation state.
For example in case of KMnO4 Mn shows +7 oxidation state.
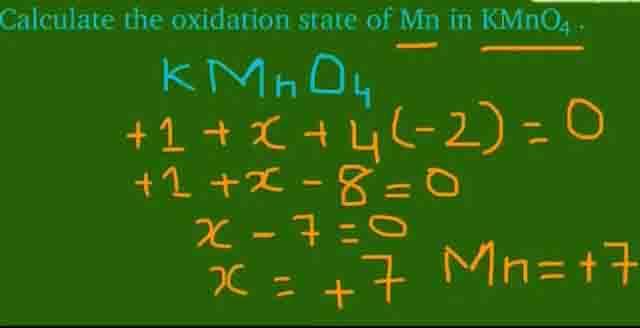
Variable oxidation states
Transition elements have greater number of electrons in d and f subshells. These can show the variation in oxidation states. It depends on the fact that which substance is going to react with that element.
Let others know about this