Written by Adeel Abbas
What are Isotopes?
Table of Contents
According to John Dalton’s point of view, all the atoms of an element were similar. However, Soddyy showed that atoms of the same element may be different.
This phenomenon of isotopy was discovered by Soddy.
Fact about isotopy
Atoms of the same element, with the same atomic number and varied atomic masses, are known as isotopes.
Definition of isotope
Wathc the video lecture for a better understanding
Properties of isotopes
- Isotopes have the same number of electrons and protons but different numbers of neutrons in their nuclei.
- The physical properties of isotopes of an element possess different physical properties because of the different number of neutrons these have in their nuclei.
- The chemical properties of isotopes of an element possess the same chemical properties because they have the same number of Valence Electrons. And it is a fact that during chemical reactions electrons are involved.
- Isotopes have the same position in the periodic table since they have the same atomic number.
Examples of isotope
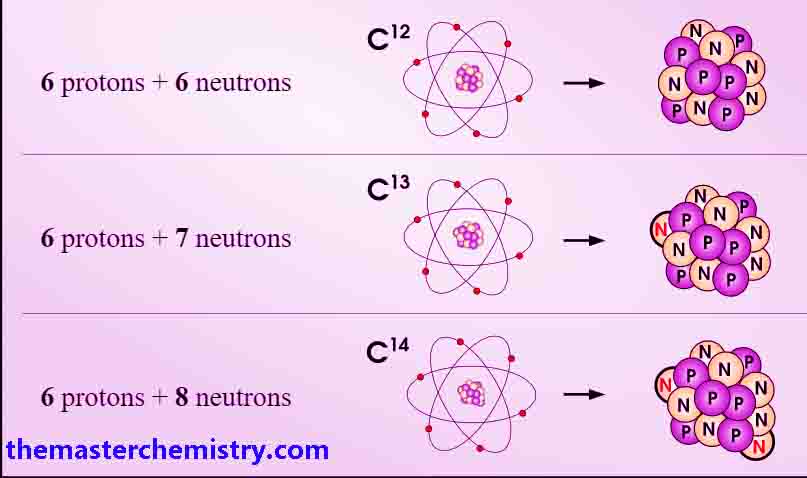
Carbon has three isotopes written as 126C, 136C, 146C and expressed as C-12, C-13, C-14, having 6, 7, and 8 neutrons in their nuclei respectively.
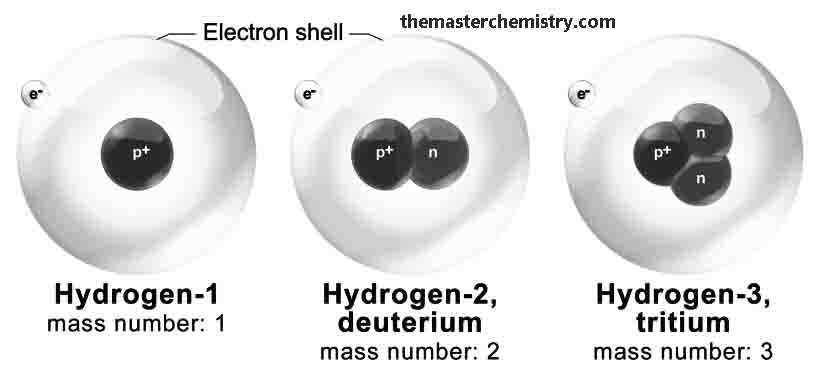
Hydrogen has three isotopes
11H Protium, 21H Deuterium 31H Tritium
Oxygen has three isotopes, Nickle has five isotopes, calcium has 6 isotopes, Palladium has 6 isotopes, cadmium has nine isotopes, tin has eleven isotopes.
Relative abundance of isotopes
The new natural occurrence of different isotopes of an element is called their relative abundance. It means which isotope of an element is found in excess that other isotopes of that element. All the elements have their own natural abundance.
The masses and abundance of isotopes of an element can be determined by the mass spectrometry.
The properties of elements are mostly like that of most abundant isotope of that element.
Facts about the relative abundance of isotopes.
There are about 280 different isotope that occur in nature.
Out of 280, there are about 40 radioactive isotopes
Besides these, about 300 unstable radioactive isotopes have been produced through artificial disintegration in nuclear reactor.
Elements with odd atomic number almost have maximum two stable isotopes.
Elements with even atomic number usually have many isotopes.
The isotope with the mass number of 4 or some multiple of 4 are more abundant.
e,g, 16O, 24Mg, 28Si, 40Ca, 56Fe form nearly 50% of the earth’s crust.
Out of 280 natural isotopes, 154 have even mass number and atomic number.
Monoisotopic elements
Elements having only one isotope are called monoisotopic elements.
Definition of monoisotopic elements
For example, arsenic, Florine, Iodine, gold, have only a single isotope.
What are radioactive isotopes?
Such atoms of the same element with different atomic masses and whose nuclei are unstable due to the nature of radiation emission in the form of alpha, beta, and gamma rays are called radioactive isotopes.
Definition of radioactive isotopes
Radioactive isotopes are also known as radioisotopes, radioactive nuclides, or radionuclides.
Let others know about this