Learning Objectives
In this article, the author has explained the position of hydrogen in the periodic table and the similarities and dissimilarities between hydrogen and other group elements.
Hydrogen a special element
Table of Contents
Hydrogen is the most important element on the planet. The position of hydrogen in the periodic table is worth discussion as its properties resemble the elements of group I-A, IV-A, and VII-A. However, it differs from these group elements as well.
Let us discuss the similarities and dissimilarities of hydrogen with these groups.
Similarities of hydrogen with group I-A elements (Alkali metals)
Hydrogen is metal like alkali metals and has one electron in the outermost shell that is s subshell.
- It also gives a positive ion by losing one electron.
- It shows the valency of one and oxidation state as +1 like alkali metals.
- Hydrogen like other alkali metals has strong tendencies to react with electronegative elements such as Cl, Br, F, O, N, etc.
- Hydrogen gives positive in ions in the aqueous solution like Na+, K+, etc.
- When electrolysis is performed for halogen acids and alkali metal halide then hydrogen and alkali metals are deposited at the cathode.
Differences of hydrogen from alkali metals
In following aspects hydrogen is different from the alkali metals…
- Hydrogen is gas but alkali metals are solids.
- Hydrogen exists in the form of diatomic molecules, but alkali metals never do so.
- Hydrogen mostly shares its electron mostly with other elements, but alkali metals always lose electrons. The reason is that hydrogen being gas and smaller in size has a higher electronegativity than alkali metals.
- In order to complete the outermost valence shell, hydrogen needs one electron, while alkali metals need seven electrons.
- Hydrogen is metal while alkali metals are metallic in nature.
- The H+ ions produced in the solution by the dissociation of acids are unstable. Therefore, it combines with H2O to give H3O+. The ions of alkali metals are stable in the solution.
Similarities of hydrogen with Halogens group VII-A
- Hydrogen is a gas at room temperature like F2, Cl2 are also gases.
- Hydrogen and halogens exist in diatomic states such as H2, F2, Cl2, Br2, I2.
- Hydrogen and halogens need one electron to form the negative ion as F-, Br-. Hydrogen also does so in the case of metallic hydrides.
- Hydrogen and halogens are non-metals.
- When the electrolysis is performed for NaH and NaCl, NaBr, NaI, etc, Hydrogen and halogens are deposited at the anode.
Difference of hydrogen from halogens
- Hydrogen has only one electron in its valence shell, while halogens have seven electrons in their valence shell.
- Hydrogen is a gas at room temperature, but Br2 and I2 are liquids solids respectively.
- The hydride ion H– is unstable in H2O, whereas X–( F–, Cl–, Br–, I–) is stable.
- Hydrogen always shows the valency of one, but halogens show variable valency.
- Hydrogen is an s-block element, but halogens are p-block elements.
Similarities of hydrogen with carbon family (VI-A)
- The outermost shell of the hydrogen and carbon family is half-filled.
- Hydrogen and the elements of the carbon family make covalent bonds with other elements.
- Hydrogen resembles with carbon family in thermodynamic properties, like ionization energy and electron affinity.
- Both hydrogen and carbon are found in organic compounds.
- Like carbon, hydrogen also shows remarkable reducing properties.
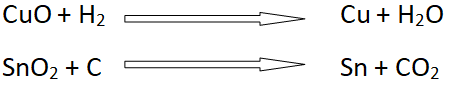
Differences of hydrogen with carbon family (IV-A)
- Hydrogen is a gas, whereas elements of group IV-A are solids at ordinary temperature and pressure.
- Hydrogen has only one electron in valence shells while members of the carbon family have four electrons in the valence shells.
- The hydrogen forms diatomic molecules, while IV-A group elements do not do so.
Let others know about this