Written by Adeel Abbas
States of the matter
Table of Contents
First of all we need to know what actually the matter is?
The matter is something that covers space and possesses mass is called matter.
Definition of Matter
There are 4 States of matter.
- Gas
- Liquid
- Solid
- Plasma
Also read: History of the periodic table
First, we will discuss the properties of gases.
Properties of gases as a state of matter

Indefinite volume
Gases have indefinite volume. The volume of a gas is equal to the volume of the container in which it is filled.
Indefinite shape
Gases have indefinite shapes because they adopt the shape of the container in which they are stored.
Low density
Gases have low densities than solids and liquids. Due to the low-density E value gases bubble true liquid and tend to rise up.
Joule Thomson effect
When a gas expands suddenly e they ka Joul effect. This effect is known as the Joule Thomson effect which is used in the process of liquefaction of gases on an industrial scale.
Effusion and diffusion
Gases have both properties to diffuse and effuse. We will cover these terms in detail letter.
Effect of temperature on gases
Gases face the expansion of volume on heating and contraction on cooling. For example, if we increase the temperature the volume of gas will also be increased and if we decrease the temperature the volume of gas will decrease. For example, boiling of water.
This property of increase in volume with the increase in temperature is somehow less common in liquid and solid.
Effect of pressure on gases
Gases expand on decreasing pressure and contract on increasing pressure.
Gas pressure
Gas molecules are constantly moving. They collide with the wall of the container and exert pressure on the walls of the container. This pressure is called gas pressure.
Let’s discuss the properties of solid
Properties of liquid as a state of matter

Definite volume of liquid
liquids have a definite volume.
Shape of liquids
Liquids have an indefinite shape and acquire the shape of the container in which they are placed.
Constant motion
Liquid molecules are set in constant motion. This motion results in the evaporation and diffusion properties of liquids.
Density of liquids
Liquids have much greater density than gases and Close to solid.
Intermolecular forces in liquids
The intermolecular forces of the liquid molecules are stronger than gases but are weaker than solid. The melting and boiling points of liquids depend upon the strength of the intermolecular forces.
Spaces in liquid molecules
The spaces among the liquid molecules are negligible just like solids.
Kinetic energy in liquid molecules
Liquid molecules have kinetic energy because there in constant motion. Liquid can be converted into solid by cooling because cooling decreases the kinetic energy of the molecules and increases the potential energy of the molecules.
Collisions among the liquid molecules
Liquid molecules collide with one another and exchange energy. These clients are just because of the kinetic energy in liquid molecules.
Diffusion in liquids
Liquid can diffuse into each other and other liquids. However the rate of diffusion is smaller than that of gases because there are stronger intermolecular forces the molecules of liquid as compared to gas molecules.
Properties of solids as the state of matter

Definite shape
Solid has a definite shape.
Definite volume
Solids have a definite volume
Spaces in solids
Atoms and molecules in solids are very close to each other which results in tight packing, therefore, fewer spaces as compared to liquids and gases.
Incompressible
Solids are incompressible due to the tight packing of atoms.
Intermolecular forces in solids
Solids have the strongest intermolecular forces. This is the reason these are tightly packed and incompressible.
Vibrational motion in Solids
Solids are tightly packed therefore they show vibrational motion when there under a force. For example, striking a solid with the hammer will cause vibrational motion in the solid.
Diffusion in solids
Solids have a negligible rate of diffusion because they are tightly packed.
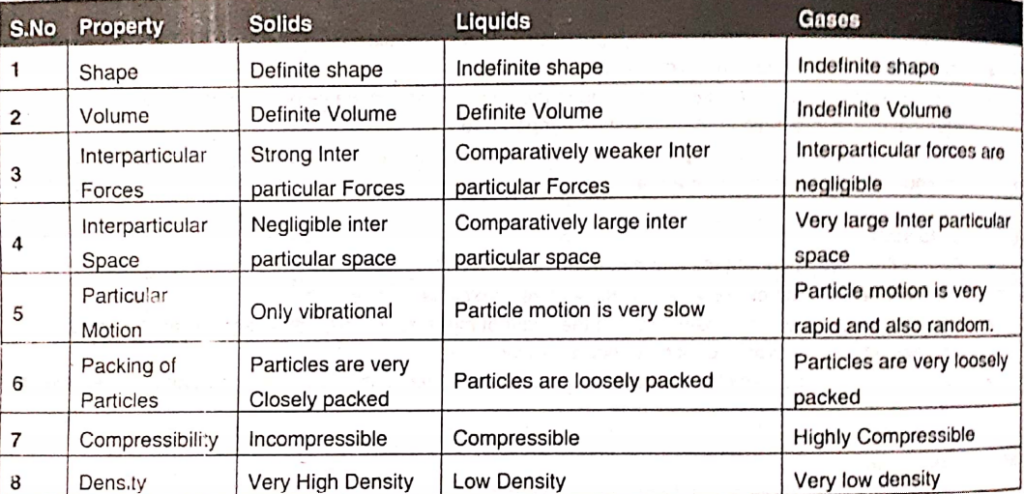
Comparison of different state of matter ( Gas, Liquid, Solid)
Plasma as a state of matter
Definition of plasma in chemistry
The state of matter which is a mixture of neutral particles, positive ions and negative electrons is called Plasma.
It is considered the 4th state of the matter.

How plasma is formed?
- When a solid is heated it is converted into liquid. When it is further heated then the liquid is converted into vapours. Therefore, the phase of matter changes from solid to liquid and then liquid to vapours.
- Now, if these vapours are further heated, then some of these lose electrons that result in positive ion formation. Hence a mixture of neutral particles that contains positive ions and negative electrons is produced. This is called plasma.
- The ionization is caused by high temperature or by radiations.
Natural and artificial plasma
Artificial plasma
It is produced by using electrical charges on a gas. For example in case of Neon signs
Plasma at low temperature is hard to maintain because outside of vacuum lower temperature plasma reacts rapidly with any molecule. Therefore, it is both useful and hard to use.
Natural plasma
It only exists at higher temperature or low temperature vacuum and does not break down or react rapidly.
The natural plasma is extremely hot over 20,000 degree centigrade minimum.
The natural plasma has so much energy that it can vaporize any material.
Occurrence of plasma
- Plasma as the 4th state is found in plenty as it is the stuff of stars. Therefore it is said that all the shining stars are plasma.
- The universe is made up of about 99% of Plasma.
- It is present everywhere in the sun and stars.
- The sun is thought to be a 1.5 million km ball of plasma that is being heated by nuclear fusion.
- The Major part of the matter is plasma in interstellar space.
- Although on planet earth, it is found in very limited amounts such as in lightning bolts, flames, auroras, and fluorescent lights.
- Its common example is the neon gas-electric bulb. When an electric current is passed through the neon gas-filled inside, it produces both plasma and light.
Properties of plasma
- It consists of a significant number of charged particles. Therefore, it responds to both electric and magnetic fields.
- The motion of particles in the plasma produces fields and electric current within plasma density. It refers to the density of charged particles.
- Plasma has a complex set of interactions despite the fact it contains ions and electrons but their number is equal.
Applications of plasma
Since plasma can respond to both electric and magnetic field it can have multiple applications.
- A fluorescent bulb totally different from a regular light bulb as it contains a long tube either straight or spiral filled with gas. When electricity is passed through the gas it charges up the gas. The plasma is produced by charging and exciting inside the bulb.
- Neon signs are glass tubes filled with gas. When electricity is passed through the tube it charges the gas and creates glowing plasma inside the tube. The color of Plasma depends upon the gas used.
- They are used for Plasma processing of semiconductors, sterilization of some medical products, lamps, laser, diamond coated films, high power microwave sources, and pulsed power switches.
- These are helpful for the generation of electricity from fusion pollution control and removal of hazardous chemicals. Thus it helps to clean up the environment.
- Plasma light up our office and homes. It helps in working of computers electronic equipment.
- Plasma drives laser and particle acceleration.
- Plasma can be used to pasteurized food.
- Plasma is used to make corrosion-resistant tools.
Futuristic approach of scientist towards during plasma
Scientists are working to use plasma effectively. For the effective use of Plasma it should satisfy two conditions.
It should have low energy
- It should survive for sufficient time without waiting and degradation.
- The applications of magnetic fields involve the use of Plasma.
Low magnetic field and low energy plasma
The magnetic field creates low-energy plasma which creates a metastable state of molecules. These will then react with another molecule with effective energy. Thus these molecules can survive long enough to react with desired molecules. These metastable particles are selective in their reactivity. Thus these can be used to solve problems like radioactive contamination.
Scientists are working on mixture of gases to use as metastable agents on plutonium and Uranium.
let others know about this