Aliphatic hydrocarbons are a significant class of organic compounds that serve as the foundational building blocks in the realm of chemistry and industry.
Aliphatic hydrocarbons are an integral part of our daily lives, finding applications in various sectors due to their versatile properties and structures.
Definition of Aliphatic Hydrocarbons
Table of Contents
Hydrocarbons are organic compounds that contain carbon and hydrogen only. The number of such compounds is very large because of the property of catenation.
Types of Aliphatic Hydrocarbons
Aliphatic hydrocarbons can be broadly categorized into two main types: saturated and unsaturated aliphatic hydrocarbons.
1. Saturated hydrocarbons
If all the valences of the carbon atom in a molecule are fully satisfied and these cannot further take up any more hydrogen atoms, then the hydrocarbons are named saturated hydrocarbons.
Examples of saturated hydrocarbons
For example Alkanes
Alkanes
Alkanes are saturated hydrocarbons composed of single carbon-carbon (C-C) and carbon-hydrogen (C-H) bonds. They exhibit a general molecular formula of CnH2n+2, where ‘n’ represents the number of carbon atoms in the chain. Common examples include methane (CH4), ethane (C2H6), propane (C3H8), and butane (C4H10).
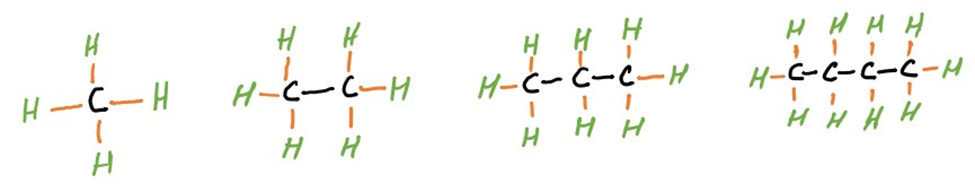
(Methane) (Ethane) (Propane) (Butane)
2. Unsaturated hydrocarbons
The compounds of carbon and hydrogen in which all four valences of carbon are not fully utilized and contain either a double or triple bond, such compounds are called unsaturated hydrocarbons.
Examples of Unsaturated hydrocarbons
For example alkenes and alkynes.
Alkenes
Alkenes are unsaturated hydrocarbons containing at least one carbon-carbon double bond (C=C). The general molecular formula for alkenes is CnH2n. Ethene (C2H4), propene (C3H6), and butene (C4H8) are common examples of alkenes.
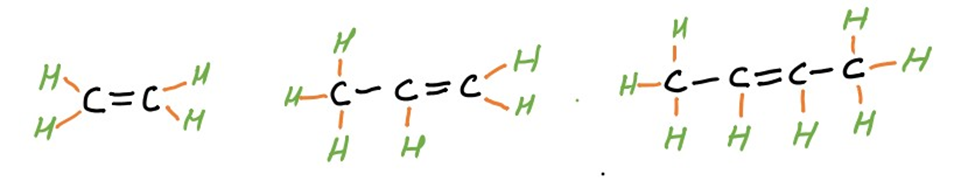
(Ethene) (Propene) (Butene)
Alkynes
Alkynes are another class of unsaturated hydrocarbons, distinguished by the presence of at least one carbon-carbon triple bond (C≡C). The general molecular formula for alkynes is CnH2n-2. Acetylene (C2H2) is a well-known example of an alkyne.
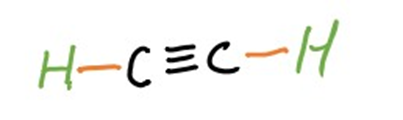
(Acetylene)
Classification of Aliphatic Hydrocarbons
Aliphatic hydrocarbons can be further classified based on their structural characteristics and properties.
1. Straight-Chain Aliphatic Hydrocarbons
Straight-chain aliphatic hydrocarbons, as the name suggests, consist of a linear arrangement of carbon atoms. They can be alkanes, alkenes, or alkynes. These hydrocarbons exhibit distinct physical and chemical properties based on the length of their carbon chains and the types of bonds present.
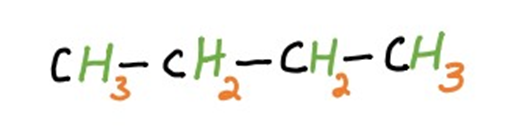
(n-butane)
2. Branched Aliphatic Hydrocarbons
Branched aliphatic hydrocarbons feature non-linear or branched carbon chains. Branching occurs when a carbon atom forms bonds with more than two other carbon atoms, leading to a complex and diverse array of structures. Branched alkanes, alkenes, and alkynes have unique properties compared to their straight-chain counterparts.
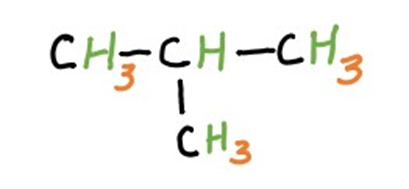
(2-Methylpropane)
3. Cycloaliphatic Hydrocarbons
Cycloaliphatic hydrocarbons form closed-ring structures, resembling aliphatic versions of aromatic compounds. These cyclic hydrocarbons can be saturated (cycloalkanes), unsaturated (cycloalkenes), or contain triple bonds (cycloalkynes). The properties of cycloaliphatic hydrocarbons vary depending on the ring size and the types of bonds present in the ring.
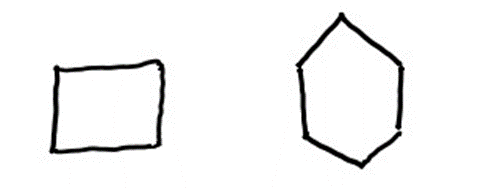
(Cyclobutane) (Cyclohexane)