Learning objectives
In this article the author has explained, what are hydrides, types of hydrides, covalent hydrides, metal hydrides, ionic hydrides, and their trends in the periodic table.
what are hydrides
Table of Contents
The binary compounds of hydrogen with other elements are known as hydrides.
Definition of hydrides
When hydrogen gets bonded with less electronegative atom than it, hydrides are formed. Normally, when hydrogen makes a bond with any elements it develops covalent character.
But when the hydrogen atom makes a bond with an element that is less electronegative than it then the ionic character is developed. Therefore, with such elements, hydrogen makes ionic hydrides.
Examples of hydrides
NaH, CaH2, B2H6, SiH4, NH3, H2S, H2O, HCl, HI, and so on are examples of hydrides.
Classification of hydrides
There are six types of hydrides which are discussed below.
- Ionic hydrides, saline hydrides, or salt-like hydrides
- Covalent hydrides
- Polymeric Hydrides
- Metallic hydrides
- Complex hydrides
- Borderline hydrides
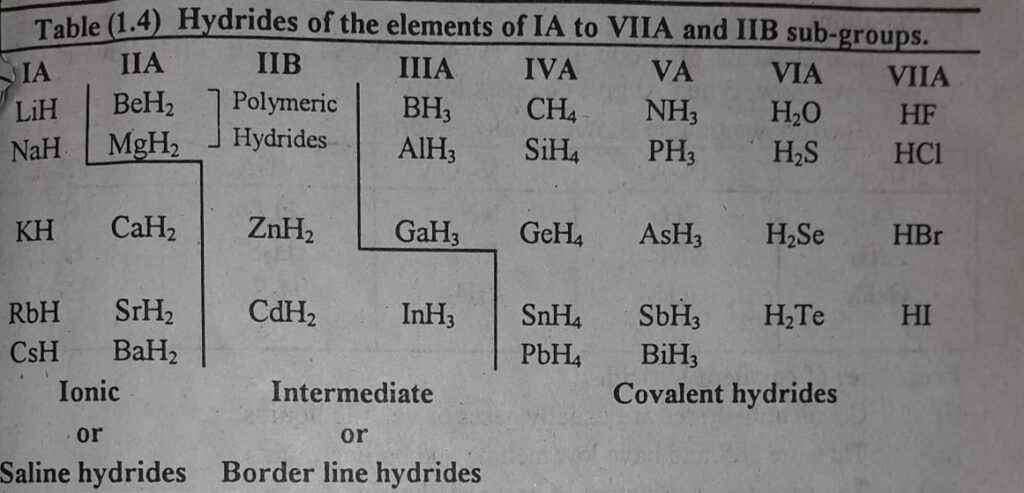
Following table clears the idea about the elements which give different types of hydrides.
Ionic hydrides
These are the hydrides of alkali metals and alkaline earth metals except Be and Mg as these give polymeric hydrides.
Definition of hydrides
For example
From group IA elements LiH, NaH, KH, RbH CsH are ionic hydrides.
From group IIA elements Be and Mg gives polymeric hydrides and CaH2, SrH2, BaH2 gives ionic hydrides.
Properties of ionic hydrides
- These are white crystalline solids
- These have high melting and boiling points
- These are good conductors of electricity in a molten state
- These have ionic bond present in them
- They are chemically reactive compounds
- These react with water to evolve hydrogen gas and give metal hydroxides
- These are thermally unstable and liberate hydrogen gas on heating
Polymeric hydrides
Be and Mg give intermediate types of hydrides. Their properties are in between ionic and covalent hydrides. These have polymeric structures and bonds are covalent in character.
Definition of polymeric hydrides
Covalent hydrides
Those hydrides which have covalent bonds present in them are called covalent hydrides.
Definition of covalent hydrides
The elements of group III-A, IV-A, V-A, and VI-A give covalent hydrides. However, B and Al give complex hydrides.
The following table shows covalent hydrides:
| III-A | IV-A | V-A | VI-A | VII-A |
| B2H6 | CH4 | NH3 | H2O | HF |
| AlH3 | SiH4 | PH3 | H2S | HCl |
| GaH3 | GeH4 | AsH3 | H2Se | HBr |
| HI |
Properties of covalent hydrides
Covalent hydrides are usually gases or volatile liquids
- These are soft
- These have low melting and boiling points
- These are non-conductors of heat and electricity
- Some of these are soluble in organic solvents
Variation of the properties of hydrides in the periodic table
Variation of hydrides in period
- The ionic character of the hydrides decreases from left to right in a period. LiH is ionic, BeH2 is polymeric and the next hydrides are of covalent character.
- The stability of covalent hydrides increases from left to right in a period.
- The possibility of the formation of hydrogen bonds increases from left to right in a period. Hydrogen bonding increases from CH4 to NH3 to H2O to HF.
Variation of hydrides in group
- The ionic character of the ionic hydrides increases down the group for group I-A and II-A.
- The stabilities of the covalent hydrides decrease from top to the bottom in a group. CH4, SiH4, is the stable hydrides, while those of Pb and bismuth Bi gives the least stable hydrides.
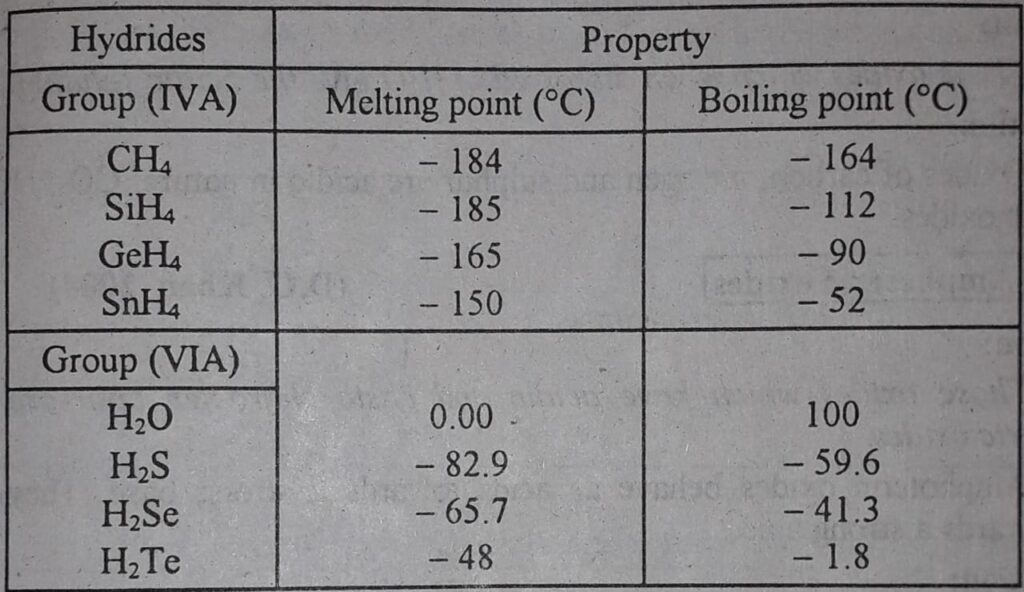
- The boiling points of the covalent hydrides generally increase down the group.
For example:
- H2O has a higher boiling point than H2S and H2Se.
- NH3 has a higher boiling point than PH3 and AsH3.
- HF has a higher boiling point than HCl and HBr.
Comparison of melting and boiling points of hydrides of group, IV-A, and VI-A
The following table shows that the greater the size of the central atom making the hydrides greater the melting and boiling point. However, the first member of every group makes such hydrides which have exceptional behavior.